The mTOR signalling pathway in human cancer
- PMID: 22408430
- PMCID: PMC3291999
- DOI: 10.3390/ijms13021886
The mTOR signalling pathway in human cancer
Abstract
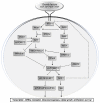
The conserved serine/threonine kinase mTOR (the mammalian target of rapamycin), a downstream effector of the PI3K/AKT pathway, forms two distinct multiprotein complexes: mTORC1 and mTORC2. mTORC1 is sensitive to rapamycin, activates S6K1 and 4EBP1, which are involved in mRNA translation. It is activated by diverse stimuli, such as growth factors, nutrients, energy and stress signals, and essential signalling pathways, such as PI3K, MAPK and AMPK, in order to control cell growth, proliferation and survival. mTORC2 is considered resistant to rapamycin and is generally insensitive to nutrients and energy signals. It activates PKC-α and AKT and regulates the actin cytoskeleton. Deregulation of multiple elements of the mTOR pathway (PI3K amplification/mutation, PTEN loss of function, AKT overexpression, and S6K1, 4EBP1 and eIF4E overexpression) has been reported in many types of cancers, particularly in melanoma, where alterations in major components of the mTOR pathway were reported to have significant effects on tumour progression. Therefore, mTOR is an appealing therapeutic target and mTOR inhibitors, including the rapamycin analogues deforolimus, everolimus and temsirolimus, are submitted to clinical trials for treating multiple cancers, alone or in combination with inhibitors of other pathways. Importantly, temsirolimus and everolimus were recently approved by the FDA for the treatment of renal cell carcinoma, PNET and giant cell astrocytoma. Small molecules that inhibit mTOR kinase activity and dual PI3K-mTOR inhibitors are also being developed. In this review, we aim to survey relevant research, the molecular mechanisms of signalling, including upstream activation and downstream effectors, and the role of mTOR in cancer, mainly in melanoma.
Keywords: cancer; mTOR; melanoma; rapamycin; therapy.
Figures

Similar articles
-
Therapeutic targeting of the mTOR-signalling pathway in cancer: benefits and limitations.Br J Pharmacol. 2014 Aug;171(16):3801-13. doi: 10.1111/bph.12749. Br J Pharmacol. 2014. PMID: 24780124 Free PMC article. Review.
-
TRAF2 and OTUD7B govern a ubiquitin-dependent switch that regulates mTORC2 signalling.Nature. 2017 May 18;545(7654):365-369. doi: 10.1038/nature22344. Epub 2017 May 10. Nature. 2017. PMID: 28489822 Free PMC article.
-
Distinct signaling mechanisms of mTORC1 and mTORC2 in glioblastoma multiforme: a tale of two complexes.Adv Biol Regul. 2015 Jan;57:64-74. doi: 10.1016/j.jbior.2014.09.004. Epub 2014 Sep 18. Adv Biol Regul. 2015. PMID: 25442674
-
Disentangling the signaling pathways of mTOR complexes, mTORC1 and mTORC2, as a therapeutic target in glioblastoma.Adv Biol Regul. 2022 Jan;83:100854. doi: 10.1016/j.jbior.2021.100854. Epub 2021 Dec 6. Adv Biol Regul. 2022. PMID: 34996736 Review.
-
Autoregulation of the mechanistic target of rapamycin (mTOR) complex 2 integrity is controlled by an ATP-dependent mechanism.J Biol Chem. 2013 Sep 20;288(38):27019-27030. doi: 10.1074/jbc.M113.498055. Epub 2013 Aug 8. J Biol Chem. 2013. PMID: 23928304 Free PMC article.
Cited by
-
Interleukin-6 expression under gravitational stress due to vibration and hypergravity in follicular thyroid cancer cells.PLoS One. 2013 Jul 2;8(7):e68140. doi: 10.1371/journal.pone.0068140. Print 2013. PLoS One. 2013. PMID: 23844163 Free PMC article.
-
mTOR Inhibition Mitigates Enhanced mRNA Translation Associated with the Metastatic Phenotype of Osteosarcoma Cells In Vivo.Clin Cancer Res. 2016 Dec 15;22(24):6129-6141. doi: 10.1158/1078-0432.CCR-16-0326. Epub 2016 Jun 24. Clin Cancer Res. 2016. PMID: 27342399 Free PMC article.
-
Differential miRNA and Protein Expression Reveals miR-1285, Its Targets TGM2 and CDH-1, as Well as CD166 and S100A13 as Potential New Biomarkers in Patients with Diabetes Mellitus and Pancreatic Adenocarcinoma.Cancers (Basel). 2024 Jul 31;16(15):2726. doi: 10.3390/cancers16152726. Cancers (Basel). 2024. PMID: 39123454 Free PMC article.
-
Nutrient signaling to mTOR and cell growth.Trends Biochem Sci. 2013 May;38(5):233-42. doi: 10.1016/j.tibs.2013.01.004. Epub 2013 Mar 1. Trends Biochem Sci. 2013. PMID: 23465396 Free PMC article. Review.
-
microRNA-17 regulates the expression of ATG7 and modulates the autophagy process, improving the sensitivity to temozolomide and low-dose ionizing radiation treatments in human glioblastoma cells.Cancer Biol Ther. 2013 Jul;14(7):574-86. doi: 10.4161/cbt.24597. Epub 2013 May 10. Cancer Biol Ther. 2013. PMID: 23792642 Free PMC article.
References
-
- Huang S., Houghton P.J. Targeting mTOR signaling for cancer therapy. Curr. Opin. Pharmacol. 2003;3:371–377. - PubMed
-
- Wullschleger S., Loewith R., Hall M.N. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. - PubMed
-
- Guertin D.A., Sabatini D.M. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. - PubMed
-
- Heitman J., Movva N.R., Hall M.N. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–909. - PubMed
-
- Sabers C.J., Martin M.M., Brunn G.J., Williams J.M., Dumont F.J., Wiederrecht G., Abraham R.T. Isolation of a protein target of the FKBP12-rapamycin complex in mammalian cells. J. Biol. Chem. 1995;270:815–822. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

