Reversible lysine acetylation regulates activity of human glycine N-acyltransferase-like 2 (hGLYATL2): implications for production of glycine-conjugated signaling molecules
- PMID: 22408254
- PMCID: PMC3351282
- DOI: 10.1074/jbc.M112.347260
Reversible lysine acetylation regulates activity of human glycine N-acyltransferase-like 2 (hGLYATL2): implications for production of glycine-conjugated signaling molecules
Abstract
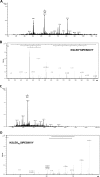
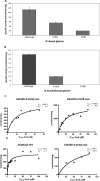
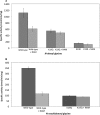
Lysine acetylation is a major post-translational modification of proteins and regulates many physiological processes such as metabolism, cell migration, aging, and inflammation. Proteomic studies have identified numerous lysine-acetylated proteins in human and mouse models (Kim, S. C., Sprung, R., Chen, Y., Xu, Y., Ball, H., Pei, J., Cheng, T., Kho, Y., Xiao, H., Xiao, L., Grishin, N. V., White, M., Yang, X. J., and Zhao, Y. (2006) Mol. Cell 23, 607-618). One family of proteins identified in this study was the murine glycine N-acyltransferase (GLYAT) enzymes, which are acetylated on lysine 19. Lysine 19 is a conserved residue in human glycine N-acyltransferase-like 2 (hGLYATL2) and in several other species, showing that this residue may be important for enzyme function. Mutation of lysine 19 in recombinant hGLYATL2 to glutamine (K19Q) and arginine (K19R) resulted in a 50-80% lower production of N-oleoyl glycine and N-arachidonoylglycine, indicating that lysine 19 is important for enzyme function. LC/MS/MS confirmed that Lys-19 is not acetylated in wild-type hGLYATL2, indicating that Lys-19 requires to be deacetylated for full activity. The hGLYATL2 enzyme conjugates medium- and long-chain saturated and unsaturated acyl-CoA esters to glycine, resulting in the production of N-oleoyl glycine and also N-arachidonoyl glycine. N-Oleoyl glycine and N-arachidonoyl glycine are structurally and functionally related to endocannabinoids and have been identified as signaling molecules that regulate functions like the perception of pain and body temperature and also have anti-inflammatory properties. In conclusion, acetylation of lysine(s) in hGLYATL2 regulates the enzyme activity, thus linking post-translational modification of proteins with the production of biological signaling molecules, the N-acyl glycines.
Figures



Similar articles
-
Identification of glycine N-acyltransferase-like 2 (GLYATL2) as a transferase that produces N-acyl glycines in humans.FASEB J. 2010 Aug;24(8):2795-803. doi: 10.1096/fj.09-148551. Epub 2010 Mar 19. FASEB J. 2010. PMID: 20305126
-
Frequent sequence variants of human glycine N-acyltransferase (GLYAT) and inborn errors of metabolism.Biochimie. 2021 Apr;183:30-34. doi: 10.1016/j.biochi.2021.02.002. Epub 2021 Feb 7. Biochimie. 2021. PMID: 33567294
-
Novel endogenous N-acyl glycines identification and characterization.Vitam Horm. 2009;81:191-205. doi: 10.1016/S0083-6729(09)81008-X. Vitam Horm. 2009. PMID: 19647113 Review.
-
Enzymatic characterization and elucidation of the catalytic mechanism of a recombinant bovine glycine N-acyltransferase.Drug Metab Dispos. 2012 Feb;40(2):346-52. doi: 10.1124/dmd.111.041657. Epub 2011 Nov 9. Drug Metab Dispos. 2012. PMID: 22071172
-
N-Acyl Amino Acids (Elmiric Acids): Endogenous Signaling Molecules with Therapeutic Potential.Mol Pharmacol. 2018 Mar;93(3):228-238. doi: 10.1124/mol.117.110841. Epub 2017 Nov 14. Mol Pharmacol. 2018. PMID: 29138268 Review.
Cited by
-
The Discovery of Novel Biomarkers Improves Breast Cancer Intrinsic Subtype Prediction and Reconciles the Labels in the METABRIC Data Set.PLoS One. 2015 Jul 1;10(7):e0129711. doi: 10.1371/journal.pone.0129711. eCollection 2015. PLoS One. 2015. PMID: 26132585 Free PMC article.
-
Insulin/IGF1-PI3K-dependent nucleolar localization of a glycolytic enzyme--phosphoglycerate mutase 2, is necessary for proper structure of nucleolus and RNA synthesis.Oncotarget. 2015 Jul 10;6(19):17237-50. doi: 10.18632/oncotarget.4044. Oncotarget. 2015. PMID: 26033454 Free PMC article.
-
Differential DNA methylation in peripheral blood mononuclear cells in adolescents exposed to significant early but not later childhood adversity.Dev Psychopathol. 2016 Nov;28(4pt2):1385-1399. doi: 10.1017/S0954579416000055. Epub 2016 Feb 5. Dev Psychopathol. 2016. PMID: 26847422 Free PMC article.
-
KAT8-catalyzed lactylation promotes eEF1A2-mediated protein synthesis and colorectal carcinogenesis.Proc Natl Acad Sci U S A. 2024 Feb 20;121(8):e2314128121. doi: 10.1073/pnas.2314128121. Epub 2024 Feb 15. Proc Natl Acad Sci U S A. 2024. PMID: 38359291 Free PMC article.
-
Acetylation mimic of lysine 280 exacerbates human Tau neurotoxicity in vivo.Sci Rep. 2016 Mar 4;6:22685. doi: 10.1038/srep22685. Sci Rep. 2016. PMID: 26940749 Free PMC article.
References
-
- Yang X. J., Seto E. (2007) HATs and HDACs. From structure function and regulation to novel strategies for therapy and prevention. Oncogene 26, 5310–5318 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

