Oxidative lipid modification of nicastrin enhances amyloidogenic γ-secretase activity in Alzheimer's disease
- PMID: 22404891
- PMCID: PMC4217088
- DOI: 10.1111/j.1474-9726.2012.00817.x
Oxidative lipid modification of nicastrin enhances amyloidogenic γ-secretase activity in Alzheimer's disease
Abstract
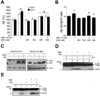
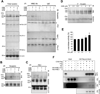
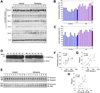
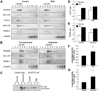
The cause of elevated level of amyloid β-peptide (Aβ42) in common late-onset sporadic [Alzheimer's disease (AD)] has not been established. Here, we show that the membrane lipid peroxidation product 4-hydroxynonenal (HNE) is associated with amyloid and neurodegenerative pathologies in AD and that it enhances γ-secretase activity and Aβ42 production in neurons. The γ-secretase substrate receptor, nicastrin, was found to be modified by HNE in cultured neurons and in brain specimens from patients with AD, in which HNE-nicastrin levels were found to be correlated with increased γ-secretase activity and Aβ plaque burden. Furthermore, HNE modification of nicastrin enhanced its binding to the γ-secretase substrate, amyloid precursor protein (APP) C99. In addition, the stimulation of γ-secretase activity and Aβ42 production by HNE were blocked by an HNE-scavenging histidine analog in a 3xTgAD mouse model of AD. These findings suggest a specific molecular mechanism by which oxidative stress increases Aβ42 production in AD and identify HNE as a novel therapeutic target upstream of the γ-secretase cleavage of APP.
© 2012 The Authors. Aging Cell © 2012 Blackwell Publishing Ltd/Anatomical Society of Great Britain and Ireland.
Figures


Similar articles
-
OCIAD2 activates γ-secretase to enhance amyloid β production by interacting with nicastrin.Cell Mol Life Sci. 2014 Jul;71(13):2561-76. doi: 10.1007/s00018-013-1515-x. Epub 2013 Nov 24. Cell Mol Life Sci. 2014. PMID: 24270855 Free PMC article.
-
Zinc and Copper Differentially Modulate Amyloid Precursor Protein Processing by γ-Secretase and Amyloid-β Peptide Production.J Biol Chem. 2017 Mar 3;292(9):3751-3767. doi: 10.1074/jbc.M116.754101. Epub 2017 Jan 17. J Biol Chem. 2017. PMID: 28096459 Free PMC article.
-
Excess of nicastrin in brain results in heterozygosity having no effect on endogenous APP processing and amyloid peptide levels in vivo.Neurobiol Dis. 2007 Feb;25(2):291-6. doi: 10.1016/j.nbd.2006.09.013. Epub 2006 Oct 27. Neurobiol Dis. 2007. PMID: 17071095
-
γ-Secretase in Alzheimer's disease.Exp Mol Med. 2022 Apr;54(4):433-446. doi: 10.1038/s12276-022-00754-8. Epub 2022 Apr 8. Exp Mol Med. 2022. PMID: 35396575 Free PMC article. Review.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
-
Oxidative stress and lipid peroxidation are upstream of amyloid pathology.Neurobiol Dis. 2015 Dec;84:109-19. doi: 10.1016/j.nbd.2015.06.013. Epub 2015 Jun 21. Neurobiol Dis. 2015. PMID: 26102023 Free PMC article.
-
Calsenilin contributes to neuronal cell death in ischemic stroke.Brain Pathol. 2013 Jul;23(4):402-12. doi: 10.1111/bpa.12013. Epub 2012 Dec 27. Brain Pathol. 2013. PMID: 23211047 Free PMC article.
-
An ECSIT-centric view of Alzheimer's disease.Bioessays. 2012 Jul;34(7):526-7. doi: 10.1002/bies.201200058. Epub 2012 May 29. Bioessays. 2012. PMID: 22641226 Free PMC article. No abstract available.
-
OCIAD2 activates γ-secretase to enhance amyloid β production by interacting with nicastrin.Cell Mol Life Sci. 2014 Jul;71(13):2561-76. doi: 10.1007/s00018-013-1515-x. Epub 2013 Nov 24. Cell Mol Life Sci. 2014. PMID: 24270855 Free PMC article.
-
Permeability transition pore-mediated mitochondrial superoxide flashes mediate an early inhibitory effect of amyloid beta1-42 on neural progenitor cell proliferation.Neurobiol Aging. 2014 May;35(5):975-89. doi: 10.1016/j.neurobiolaging.2013.11.002. Epub 2013 Nov 13. Neurobiol Aging. 2014. PMID: 24325797 Free PMC article.
References
-
- Arumugam TV, Chan SL, Jo DG, Yilmaz G, Tang SC, Cheng A, Gleichmann M, Okun E, Dixit VD, Chigurupati S, Mughal MR, Ouyang X, Miele L, Magnus T, Poosala S, Granger DN, Mattson MP. Gamma secretase-mediated Notch signaling worsens brain damage and functional outcome in ischemic stroke. Nat Med. 2006;12:621–623. - PubMed
-
- Bonda DJ, Wang X, Perry G, Nunomura A, Tabaton M, Zhu X, Smith MA. Oxidative stress in Alzheimer disease: A possibility for prevention. Neuropharmacology. 2010 - PubMed
-
- Farmery MR, Tjernberg LO, Pursglove SE, Bergman A, Winblad B, Naslund J. Partial purification and characterization of gamma-secretase from post-mortem human brain. J Biol Chem. 2003;278:24277–24284. - PubMed
-
- Fraering PC, Ye W, Strub JM, Dolios G, LaVoie MJ, Ostaszewski BL, van Dorsselaer A, Wang R, Selkoe DJ, Wolfe MS. Purification and characterization of the human gamma-secretase complex. Biochemistry. 2004;43:9774–9789. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

