TCR gene transfer: MAGE-C2/HLA-A2 and MAGE-A3/HLA-DP4 epitopes as melanoma-specific immune targets
- PMID: 22400038
- PMCID: PMC3287115
- DOI: 10.1155/2012/586314
TCR gene transfer: MAGE-C2/HLA-A2 and MAGE-A3/HLA-DP4 epitopes as melanoma-specific immune targets
Abstract
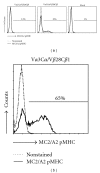
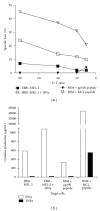
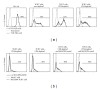
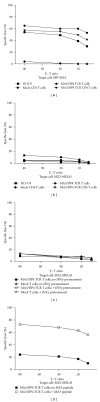
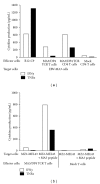
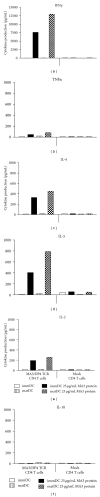
Adoptive therapy with TCR gene-engineered T cells provides an attractive and feasible treatment option for cancer patients. Further development of TCR gene therapy requires the implementation of T-cell target epitopes that prevent "on-target" reactivity towards healthy tissues and at the same time direct a clinically effective response towards tumor tissues. Candidate epitopes that meet these criteria are MAGE-C2(336-344)/HLA-A2 (MC2/A2) and MAGE-A3(243-258)/HLA-DP4 (MA3/DP4). We molecularly characterized TCRαβ genes of an MC2/A2-specific CD8 and MA3/DP4-specific CD4 T-cell clone derived from melanoma patients who responded clinically to MAGE vaccination. We identified MC2/A2 and MA3/DP4-specific TCR-Vα3/Vβ28 and TCR-Vα38/Vβ2 chains and validated these TCRs in vitro upon gene transfer into primary human T cells. The MC2 and MA3 TCR were surface-expressed and mediated CD8 T-cell functions towards melanoma cell lines and CD4 T-cell functions towards dendritic cells, respectively. We intend to start testing these MAGE-specific TCRs in phase I clinical trial.
Figures

Similar articles
-
A TCR targeting the HLA-A*0201-restricted epitope of MAGE-A3 recognizes multiple epitopes of the MAGE-A antigen superfamily in several types of cancer.J Immunol. 2011 Jan 15;186(2):685-96. doi: 10.4049/jimmunol.1001775. Epub 2010 Dec 13. J Immunol. 2011. PMID: 21149604 Free PMC article.
-
A polyclonal anti-vaccine CD4 T cell response detected with HLA-DP4 multimers in a melanoma patient vaccinated with MAGE-3.DP4-peptide-pulsed dendritic cells.Eur J Immunol. 2005 Apr;35(4):1066-75. doi: 10.1002/eji.200425847. Eur J Immunol. 2005. PMID: 15756643
-
Redirecting human CD4+ T lymphocytes to the MHC class I-restricted melanoma antigen MAGE-A1 by TCR alphabeta gene transfer requires CD8alpha.Gene Ther. 2005 Jan;12(2):140-6. doi: 10.1038/sj.gt.3302388. Gene Ther. 2005. PMID: 15496961
-
Potential use of T cell receptor genes to modify hematopoietic stem cells for the gene therapy of cancer.Pathol Oncol Res. 1999;5(1):3-15. doi: 10.1053/paor.1999.0003. Pathol Oncol Res. 1999. PMID: 10079371 Review.
-
Dendritic cell vaccination and immune monitoring.Cancer Immunol Immunother. 2008 Oct;57(10):1559-68. doi: 10.1007/s00262-008-0553-y. Epub 2008 Jul 10. Cancer Immunol Immunother. 2008. PMID: 18618110 Free PMC article. Review.
Cited by
-
Engineered cytotoxic T lymphocytes with AFP-specific TCR gene for adoptive immunotherapy in hepatocellular carcinoma.Tumour Biol. 2016 Jan;37(1):799-806. doi: 10.1007/s13277-015-3845-9. Epub 2015 Aug 7. Tumour Biol. 2016. PMID: 26250457
-
Gene Engineering T Cells with T-Cell Receptor for Adoptive Therapy.Methods Mol Biol. 2022;2453:209-229. doi: 10.1007/978-1-0716-2115-8_13. Methods Mol Biol. 2022. PMID: 35622329 Free PMC article.
-
A high-affinity human TCR-like antibody detects celiac disease gluten peptide-MHC complexes and inhibits T cell activation.Sci Immunol. 2021 Aug 20;6(62):eabg4925. doi: 10.1126/sciimmunol.abg4925. Sci Immunol. 2021. PMID: 34417258 Free PMC article.
-
Expression and prognostic relevance of MAGE-A3 and MAGE-C2 in non-small cell lung cancer.Oncol Lett. 2017 Mar;13(3):1609-1618. doi: 10.3892/ol.2017.5665. Epub 2017 Feb 1. Oncol Lett. 2017. PMID: 28454298 Free PMC article.
-
Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity.Mol Ther. 2013 Apr;21(4):904-12. doi: 10.1038/mt.2013.17. Epub 2013 Feb 19. Mol Ther. 2013. PMID: 23423337 Free PMC article. Clinical Trial.
References
-
- Yee C, Thompson JA, Byrd D, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(25):16168–16173. - PMC - PubMed
-
- Walter EA, Greenberg PD, Gilbert MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. The New England Journal of Medicine. 1995;333(16):1038–1044. - PubMed
-
- Savoldo B, Heslop HE, Rooney CM. The use of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus induced lymphoma in transplant recipients. Leukemia and Lymphoma. 2000;39(5-6):455–464. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

