Distinct acyl protein transferases and thioesterases control surface expression of calcium-activated potassium channels
- PMID: 22399288
- PMCID: PMC3340283
- DOI: 10.1074/jbc.M111.335547
Distinct acyl protein transferases and thioesterases control surface expression of calcium-activated potassium channels
Abstract
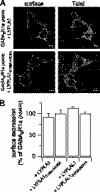
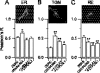
Protein palmitoylation is rapidly emerging as an important determinant in the regulation of ion channels, including large conductance calcium-activated potassium (BK) channels. However, the enzymes that control channel palmitoylation are largely unknown. Indeed, although palmitoylation is the only reversible lipid modification of proteins, acyl thioesterases that control ion channel depalmitoylation have not been identified. Here, we demonstrate that palmitoylation of the intracellular S0-S1 loop of BK channels is controlled by two of the 23 mammalian palmitoyl-transferases, zDHHC22 and zDHHC23. Palmitoylation by these acyl transferases is essential for efficient cell surface expression of BK channels. In contrast, depalmitoylation is controlled by the cytosolic thioesterase APT1 (LYPLA1), but not APT2 (LYPLA2). In addition, we identify a splice variant of LYPLAL1, a homolog with ∼30% identity to APT1, that also controls BK channel depalmitoylation. Thus, both palmitoyl acyltransferases and acyl thioesterases display discrete substrate specificity for BK channels. Because depalmitoylated BK channels are retarded in the trans-Golgi network, reversible protein palmitoylation provides a critical checkpoint to regulate exit from the trans-Golgi network and thus control BK channel cell surface expression.
Figures

Similar articles
-
Palmitoylation of the S0-S1 linker regulates cell surface expression of voltage- and calcium-activated potassium (BK) channels.J Biol Chem. 2010 Oct 22;285(43):33307-33314. doi: 10.1074/jbc.M110.153940. Epub 2010 Aug 6. J Biol Chem. 2010. PMID: 20693285 Free PMC article.
-
Site-specific deacylation by ABHD17a controls BK channel splice variant activity.J Biol Chem. 2020 Dec 4;295(49):16487-16496. doi: 10.1074/jbc.RA120.015349. Epub 2020 Sep 10. J Biol Chem. 2020. PMID: 32913120 Free PMC article.
-
Palmitoylation of the β4-subunit regulates surface expression of large conductance calcium-activated potassium channel splice variants.J Biol Chem. 2013 May 3;288(18):13136-44. doi: 10.1074/jbc.M113.461830. Epub 2013 Mar 16. J Biol Chem. 2013. PMID: 23504458 Free PMC article.
-
Enzymatic protein depalmitoylation by acyl protein thioesterases.Biochem Soc Trans. 2015 Apr;43(2):193-8. doi: 10.1042/BST20140235. Biochem Soc Trans. 2015. PMID: 25849916 Review.
-
S-acylation dependent post-translational cross-talk regulates large conductance calcium- and voltage- activated potassium (BK) channels.Front Physiol. 2014 Aug 5;5:281. doi: 10.3389/fphys.2014.00281. eCollection 2014. Front Physiol. 2014. PMID: 25140154 Free PMC article. Review.
Cited by
-
Fine-mapping and genetic analysis of the loci affecting hepatic iron overload in mice.PLoS One. 2013 May 10;8(5):e63280. doi: 10.1371/journal.pone.0063280. Print 2013. PLoS One. 2013. PMID: 23675470 Free PMC article.
-
A systematic analysis of protein palmitoylation in Caenorhabditis elegans.BMC Genomics. 2014 Oct 2;15(1):841. doi: 10.1186/1471-2164-15-841. BMC Genomics. 2014. PMID: 25277130 Free PMC article.
-
Regulatory effects of protein S-acylation on insulin secretion and insulin action.Open Biol. 2021 Mar;11(3):210017. doi: 10.1098/rsob.210017. Epub 2021 Mar 31. Open Biol. 2021. PMID: 33784857 Free PMC article. Review.
-
Stochastic palmitoylation of accessible cysteines in membrane proteins revealed by native mass spectrometry.Nat Commun. 2017 Nov 3;8(1):1280. doi: 10.1038/s41467-017-01461-z. Nat Commun. 2017. PMID: 29097667 Free PMC article.
-
Dynamic palmitoylation links cytosol-membrane shuttling of acyl-protein thioesterase-1 and acyl-protein thioesterase-2 with that of proto-oncogene H-ras product and growth-associated protein-43.J Biol Chem. 2013 Mar 29;288(13):9112-25. doi: 10.1074/jbc.M112.421073. Epub 2013 Feb 8. J Biol Chem. 2013. PMID: 23396970 Free PMC article.
References
-
- Linder M. E., Deschenes R. J. (2007) Palmitoylation. Policing protein stability and traffic. Nat. Rev. Mol. Cell Biol. 8, 74–84 - PubMed
-
- Fukata Y., Fukata M. (2010) Protein palmitoylation in neuronal development and synaptic plasticity. Nat. Rev. Neurosci. 11, 161–175 - PubMed
-
- Greaves J., Chamberlain L. H. (2011) DHHC palmitoyl transferases. Substrate interactions and (patho)physiology. Trends Biochem. Sci. 36, 245–253 - PubMed
-
- Zeidman R., Jackson C. S., Magee A. I. (2009) Protein acyl thioesterases. Mol. Membr. Biol. 26, 32–41 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

