Predicting the F(ab)-mediated effect of monoclonal antibodies in vivo by combining cell-level kinetic and pharmacokinetic modelling
- PMID: 22399130
- PMCID: PMC3333800
- DOI: 10.1007/s10928-012-9243-7
Predicting the F(ab)-mediated effect of monoclonal antibodies in vivo by combining cell-level kinetic and pharmacokinetic modelling
Abstract
Cell-level kinetic models for therapeutically relevant processes increasingly benefit the early stages of drug development. Later stages of the drug development processes, however, rely on pharmacokinetic compartment models while cell-level dynamics are typically neglected. We here present a systematic approach to integrate cell-level kinetic models and pharmacokinetic compartment models. Incorporating target dynamics into pharmacokinetic models is especially useful for the development of therapeutic antibodies because their effect and pharmacokinetics are inherently interdependent. The approach is illustrated by analysing the F(ab)-mediated inhibitory effect of therapeutic antibodies targeting the epidermal growth factor receptor. We build a multi-level model for anti-EGFR antibodies by combining a systems biology model with in vitro determined parameters and a pharmacokinetic model based on in vivo pharmacokinetic data. Using this model, we investigated in silico the impact of biochemical properties of anti-EGFR antibodies on their F(ab)-mediated inhibitory effect. The multi-level model suggests that the F(ab)-mediated inhibitory effect saturates with increasing drug-receptor affinity, thereby limiting the impact of increasing antibody affinity on improving the effect. This indicates that observed differences in the therapeutic effects of high affinity antibodies in the market and in clinical development may result mainly from Fc-mediated indirect mechanisms such as antibody-dependent cell cytotoxicity.
Figures


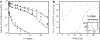

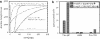
Comment in
-
Evolution of Antibody-Drug Conjugate Tumor Disposition Model to Predict Preclinical Tumor Pharmacokinetics of Trastuzumab-Emtansine (T-DM1).AAPS J. 2016 Jul;18(4):861-75. doi: 10.1208/s12248-016-9904-3. Epub 2016 Mar 30. AAPS J. 2016. PMID: 27029797 Free PMC article.
Similar articles
-
A comparison of the in vitro and in vivo activities of IgG and F(ab')2 fragments of a mixture of three monoclonal anti-Her-2 antibodies.Clin Cancer Res. 2004 May 15;10(10):3542-51. doi: 10.1158/1078-0432.CCR-03-0549. Clin Cancer Res. 2004. PMID: 15161714
-
Optimization of IGF-1R SPECT/CT imaging using 111In-labeled F(ab')2 and Fab fragments of the monoclonal antibody R1507.Mol Pharm. 2012 Aug 6;9(8):2314-21. doi: 10.1021/mp300232n. Epub 2012 Jul 13. Mol Pharm. 2012. PMID: 22747077
-
A monoclonal antibody against hinge-cleaved IgG restores effector function to proteolytically-inactivated IgGs in vitro and in vivo.MAbs. 2014;6(5):1265-73. doi: 10.4161/mabs.29825. Epub 2014 Oct 30. MAbs. 2014. PMID: 25517311 Free PMC article.
-
Are endosomal trafficking parameters better targets for improving mAb pharmacokinetics than FcRn binding affinity?Mol Immunol. 2013 Dec;56(4):660-74. doi: 10.1016/j.molimm.2013.05.008. Epub 2013 Aug 2. Mol Immunol. 2013. PMID: 23917469 Review.
-
Full-length recombinant antibodies from Escherichia coli: production, characterization, effector function (Fc) engineering, and clinical evaluation.MAbs. 2022 Jan-Dec;14(1):2111748. doi: 10.1080/19420862.2022.2111748. MAbs. 2022. PMID: 36018829 Free PMC article. Review.
Cited by
-
Bench to bedside translation of antibody drug conjugates using a multiscale mechanistic PK/PD model: a case study with brentuximab-vedotin.J Pharmacokinet Pharmacodyn. 2012 Dec;39(6):643-59. doi: 10.1007/s10928-012-9276-y. Epub 2012 Nov 15. J Pharmacokinet Pharmacodyn. 2012. PMID: 23151991
-
Impact of altered endogenous IgG on unspecific mAb clearance.J Pharmacokinet Pharmacodyn. 2017 Aug;44(4):351-374. doi: 10.1007/s10928-017-9524-2. Epub 2017 Apr 24. J Pharmacokinet Pharmacodyn. 2017. PMID: 28439684
-
A Tutorial on Target-Mediated Drug Disposition (TMDD) Models.CPT Pharmacometrics Syst Pharmacol. 2015 Jun;4(6):324-37. doi: 10.1002/psp4.41. Epub 2015 Jun 15. CPT Pharmacometrics Syst Pharmacol. 2015. PMID: 26225261 Free PMC article.
-
A cell-level model of pharmacodynamics-mediated drug disposition.J Pharmacokinet Pharmacodyn. 2016 Oct;43(5):513-27. doi: 10.1007/s10928-016-9491-z. Epub 2016 Sep 9. J Pharmacokinet Pharmacodyn. 2016. PMID: 27612462
-
Cumulative signal transmission in nonlinear reaction-diffusion networks.PLoS One. 2013 May 8;8(5):e62834. doi: 10.1371/journal.pone.0062834. Print 2013. PLoS One. 2013. PMID: 23667528 Free PMC article.
References
-
- Meibohm B. The role of pharmacokinetics and pharmacodynamics in the development of biotech drugs. In: Meibohm B, editor. Pharmacokinetics and pharmacodynamics of biotech drugs: principles and case studies in drug development. Weinheim: Wiley-VCH; 2007.
-
- Mould DR, Sweeney KRD. The pharmacokinetics and pharmacodynamics of monoclonal antibodies–mechanistic modeling applied to drug development. Curr Opin Drug Discov Devel. 2007;10:84–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

