Targeting of the non-mutated tumor antigen HER2/neu to mature dendritic cells induces an integrated immune response that protects against breast cancer in mice
- PMID: 22397502
- PMCID: PMC3446373
- DOI: 10.1186/bcr3135
Targeting of the non-mutated tumor antigen HER2/neu to mature dendritic cells induces an integrated immune response that protects against breast cancer in mice
Abstract
Introduction: Given their relative simplicity of manufacture and ability to be injected repeatedly, vaccines in a protein format are attractive for breast and other cancers. However, soluble human epidermal growth factor receptor (HER2)/neu protein as a vaccine has not been immunogenic. When protein is directly targeted to antigen uptake receptors, such as DEC205 (DEC), efficient processing and presentation of antigen take place. The aim of this study was to determine the immunogenicity of a HER2 protein vaccine that directly targets to DEC+ dendritic cells (DCs) in a mouse breast cancer model.
Methods: We genetically engineered the HER2 extracellular domain into a monoclonal antibody specific for DEC (DEC-HER2). Mice of various genetic backgrounds were immunized with DEC-HER2 in combination with DC maturation stimuli (poly IC ± CD40 Ab). Vaccine-induced T cell immunity was determined by analyzing the ability of CD4+/CD8+ T cell to produce interferon (IFN)-gamma and proliferate upon antigen rechallenge. Sera were assessed for the presence of antigen specific antibody (Ab). For vaccine efficacy, FVB/N mice were immunized with DEC-HER2 in combination with poly IC and protection against neu-expressing mammary tumors was assessed. Protection mechanisms and tumor-specific T cell responses were also evaluated.
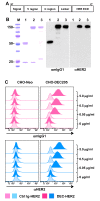
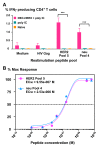
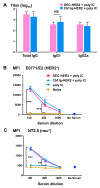
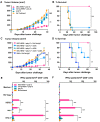
Results: We demonstrate that DEC-HER2 fusion mAb, but not Ctrl Ig-HER2, elicits strong, broad and multifunctional CD4+ T cell immunity, CD8+ T cell responses, and humoral immunity specific for HER2 antigen. Cross-reactivity to rat neu protein was also observed. Importantly, mice xeno-primed with DEC-HER2 were protected from a neu-expressing mammary tumor challenge. Both CD4+ and CD8+ T cells mediated the tumor protection. Robust anti-tumor T cell immunity was detected in tumor protected mice.
Conclusions: Immunization of mice with HER2 protein vaccine targeting DEC+ DCs in vivo induced high levels of T- and B-cell immunity. Non-targeted HER2 protein was poorly immunogenic for CD4+ and CD8+ T cells. This vaccination approach provided long-term survival benefit for mice challenged with neu-expressing tumor following as little as 2.7 μg of HER2 protein incorporated in the vaccine. Vaccine-induced CD4+ and CD8+ T cells were both essential for tumor protection. This immunization strategy demonstrates great potential towards the development of vaccines for breast cancer patients.
Figures



Similar articles
-
Potent CD4+ T-cell epitope P30 enhances HER2/neu-engineered dendritic cell-induced immunity against Tg1-1 breast cancer in transgenic FVBneuN mice by enhanced CD4+ T-cell-stimulated CTL responses.Cancer Gene Ther. 2013 Oct;20(10):590-8. doi: 10.1038/cgt.2013.60. Epub 2013 Sep 20. Cancer Gene Ther. 2013. PMID: 24052129
-
HSP110-HER2/neu chaperone complex vaccine induces protective immunity against spontaneous mammary tumors in HER-2/neu transgenic mice.J Immunol. 2003 Oct 15;171(8):4054-61. doi: 10.4049/jimmunol.171.8.4054. J Immunol. 2003. PMID: 14530326
-
Exosomal pMHC-I complex targets T cell-based vaccine to directly stimulate CTL responses leading to antitumor immunity in transgenic FVBneuN and HLA-A2/HER2 mice and eradicating trastuzumab-resistant tumor in athymic nude mice.Breast Cancer Res Treat. 2013 Jul;140(2):273-84. doi: 10.1007/s10549-013-2626-7. Epub 2013 Jul 24. Breast Cancer Res Treat. 2013. PMID: 23881522
-
Expansion of HER2/neu-specific T cells ex vivo following immunization with a HER2/neu peptide-based vaccine.Clin Breast Cancer. 2001 Apr;2(1):73-9. doi: 10.3816/CBC.2001.n.014. Clin Breast Cancer. 2001. PMID: 11899386 Review.
-
Dendritic cell-targeted protein vaccines: a novel approach to induce T-cell immunity.J Intern Med. 2012 Feb;271(2):183-92. doi: 10.1111/j.1365-2796.2011.02496.x. Epub 2012 Jan 4. J Intern Med. 2012. PMID: 22126373 Free PMC article. Review.
Cited by
-
Dendritic cell-based vaccination in cancer: therapeutic implications emerging from murine models.Front Immunol. 2015 May 20;6:243. doi: 10.3389/fimmu.2015.00243. eCollection 2015. Front Immunol. 2015. PMID: 26042126 Free PMC article. Review.
-
Human XCR1+ dendritic cells derived in vitro from CD34+ progenitors closely resemble blood dendritic cells, including their adjuvant responsiveness, contrary to monocyte-derived dendritic cells.J Immunol. 2014 Aug 15;193(4):1622-35. doi: 10.4049/jimmunol.1401243. Epub 2014 Jul 9. J Immunol. 2014. PMID: 25009205 Free PMC article.
-
Strategy for identifying dendritic cell-processed CD4+ T cell epitopes from the HIV gag p24 protein.PLoS One. 2012;7(7):e41897. doi: 10.1371/journal.pone.0041897. Epub 2012 Jul 30. PLoS One. 2012. PMID: 22860026 Free PMC article.
-
Recent Progress in Dendritic Cell-Based Cancer Immunotherapy.Cancers (Basel). 2021 May 20;13(10):2495. doi: 10.3390/cancers13102495. Cancers (Basel). 2021. PMID: 34065346 Free PMC article. Review.
-
Antigen targeting to dendritic cells allows the identification of a CD4 T-cell epitope within an immunodominant Trypanosoma cruzi antigen.PLoS One. 2015 Feb 13;10(2):e0117778. doi: 10.1371/journal.pone.0117778. eCollection 2015. PLoS One. 2015. PMID: 25679777 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

