Evolution of the voltage sensor domain of the voltage-sensitive phosphoinositide phosphatase VSP/TPTE suggests a role as a proton channel in eutherian mammals
- PMID: 22396523
- PMCID: PMC3424408
- DOI: 10.1093/molbev/mss083
Evolution of the voltage sensor domain of the voltage-sensitive phosphoinositide phosphatase VSP/TPTE suggests a role as a proton channel in eutherian mammals
Abstract
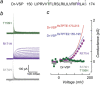
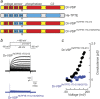
The voltage-sensitive phosphoinositide phosphatases provide a mechanism to couple changes in the transmembrane electrical potential to intracellular signal transduction pathways. These proteins share a domain architecture that is conserved in deuterostomes. However, gene duplication events in primates, including humans, give rise to the paralogs TPTE and TPTE2 that retain protein domain organization but, in the case of TPTE, have lost catalytic activity. Here, we present evidence that these human proteins contain a functional voltage sensor, similar to that in nonmammalian orthologs. However, domains of these human proteins can also generate a noninactivating outward current that is not observed in zebra fish or tunicate orthologs. This outward current has the anticipated characteristics of a voltage-sensitive proton current and is due to the appearance of a single histidine residue in the S4 transmembrane segment of the voltage sensor. Histidine is observed at this position only during the eutherian radiation. Domains from both human paralogs generate proton currents. This apparent gain of proton channel function during the evolution of the TPTE protein family may account for the conservation of voltage sensor domains despite the loss of phosphatase activity in some human paralogs.
Figures



Similar articles
-
Coupling between the voltage-sensing and phosphatase domains of Ci-VSP.J Gen Physiol. 2009 Jul;134(1):5-14. doi: 10.1085/jgp.200910215. J Gen Physiol. 2009. PMID: 19564425 Free PMC article.
-
Depolarization activates the phosphoinositide phosphatase Ci-VSP, as detected in Xenopus oocytes coexpressing sensors of PIP2.J Physiol. 2007 Sep 15;583(Pt 3):875-89. doi: 10.1113/jphysiol.2007.134775. Epub 2007 Jul 5. J Physiol. 2007. PMID: 17615106 Free PMC article.
-
Interaction between S4 and the phosphatase domain mediates electrochemical coupling in voltage-sensing phosphatase (VSP).Proc Natl Acad Sci U S A. 2022 Jun 28;119(26):e2200364119. doi: 10.1073/pnas.2200364119. Epub 2022 Jun 21. Proc Natl Acad Sci U S A. 2022. PMID: 35733115 Free PMC article.
-
Phosphoinositide phosphatases: emerging roles as voltage sensors?Mol Interv. 2005 Oct;5(5):274-7. doi: 10.1124/mi.5.5.5. Mol Interv. 2005. PMID: 16249522 Review.
-
Voltage-sensing phosphatase: its molecular relationship with PTEN.Physiology (Bethesda). 2011 Feb;26(1):6-13. doi: 10.1152/physiol.00035.2010. Physiology (Bethesda). 2011. PMID: 21357898 Review.
Cited by
-
Genome-Wide Search for Tyrosine Phosphatases in the Human Genome Through Computational Approaches Leads to the Discovery of Few New Domain Architectures.Evol Bioinform Online. 2019 Apr 9;15:1176934319840289. doi: 10.1177/1176934319840289. eCollection 2019. Evol Bioinform Online. 2019. PMID: 31007525 Free PMC article.
-
Exploring Flexibility and Folding Patterns Throughout Time in Voltage Sensors.J Mol Evol. 2023 Dec;91(6):819-836. doi: 10.1007/s00239-023-10140-1. Epub 2023 Nov 13. J Mol Evol. 2023. PMID: 37955698
-
Voltage-Controlled Enzymes: The New JanusBifrons.Front Pharmacol. 2012 Sep 13;3:161. doi: 10.3389/fphar.2012.00161. eCollection 2012. Front Pharmacol. 2012. PMID: 22993507 Free PMC article.
-
Voltage-gated proton channels: molecular biology, physiology, and pathophysiology of the H(V) family.Physiol Rev. 2013 Apr;93(2):599-652. doi: 10.1152/physrev.00011.2012. Physiol Rev. 2013. PMID: 23589829 Free PMC article. Review.
-
Sensing charges of the Ciona intestinalis voltage-sensing phosphatase.J Gen Physiol. 2013 Nov;142(5):543-55. doi: 10.1085/jgp.201310993. Epub 2013 Oct 14. J Gen Physiol. 2013. PMID: 24127524 Free PMC article.
References
-
- Armstrong CM, Bezanilla F. Currents related to movement of the gating particles of the sodium channels. Nature. 1973;242:459–461. - PubMed
-
- Balla T. Phosphoinositide-derived messengers in endocrine signaling. J Endocrinol. 2006;188:135–153. - PubMed
-
- Bezanilla F. How membrane proteins sense voltage. Nat Rev Mol Cell Biol. 2008;9:323–332. - PubMed
-
- Breckenridge LJ, Almers W. Currents through the fusion pore that forms during exocytosis of a secretory vesicle. Nature. 1987;328:814–817. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

