Prime-boost vaccination with rBCG/rAd35 enhances CD8⁺ cytolytic T-cell responses in lesions from Mycobacterium tuberculosis-infected primates
- PMID: 22396020
- PMCID: PMC3388130
- DOI: 10.2119/molmed.2011.00222
Prime-boost vaccination with rBCG/rAd35 enhances CD8⁺ cytolytic T-cell responses in lesions from Mycobacterium tuberculosis-infected primates
Abstract
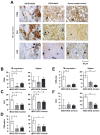
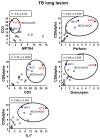
To prevent the global spread of tuberculosis (TB) infection, a novel vaccine that triggers potent and long-lived immunity is urgently required. A plasmid-based vaccine has been developed to enhance activation of major histocompatibility complex (MHC) class I-restricted CD8⁺ cytolytic T cells using a recombinant Bacille Calmette-Guérin (rBCG) expressing a pore-forming toxin and the Mycobacterium tuberculosis (Mtb) antigens Ag85A, 85B and TB10.4 followed by a booster with a nonreplicating adenovirus 35 (rAd35) vaccine vector encoding the same Mtb antigens. Here, the capacity of the rBCG/rAd35 vaccine to induce protective and biologically relevant CD8⁺ T-cell responses in a nonhuman primate model of TB was investigated. After prime/boost immunizations and challenge with virulent Mtb in rhesus macaques, quantification of immune responses at the single-cell level in cryopreserved tissue specimen from infected organs was performed using in situ computerized image analysis as a technological platform. Significantly elevated levels of CD3⁺ and CD8⁺ T cells as well as cells expressing interleukin (IL)-7, perforin and granulysin were found in TB lung lesions and spleen from rBCG/rAd35-vaccinated animals compared with BCG/rAd35-vaccinated or unvaccinated animals. The local increase in CD8⁺ cytolytic T cells correlated with reduced expression of the Mtb antigen MPT64 and also with prolonged survival after the challenge. Our observations suggest that a protective immune response in rBCG/rAd35-vaccinated nonhuman primates was associated with enhanced MHC class I antigen presentation and activation of CD8⁺ effector T-cell responses at the local site of infection in Mtb-challenged animals.
Figures




Similar articles
-
MHC-restricted Ag85B-specific CD8+ T cells are enhanced by recombinant BCG prime and DNA boost immunization in mice.Eur J Immunol. 2019 Sep;49(9):1399-1414. doi: 10.1002/eji.201847988. Epub 2019 Jun 19. Eur J Immunol. 2019. PMID: 31135967 Free PMC article.
-
The Induction of Antigen 85B-Specific CD8+ T Cells by Recombinant BCG Protects against Mycobacterial Infection in Mice.Int J Mol Sci. 2023 Jan 4;24(2):966. doi: 10.3390/ijms24020966. Int J Mol Sci. 2023. PMID: 36674484 Free PMC article.
-
Efficacy of recombinant bacille Calmette-Guérin vaccine secreting interleukin-15/antigen 85B fusion protein in providing protection against Mycobacterium tuberculosis.J Infect Dis. 2008 May 1;197(9):1263-74. doi: 10.1086/586902. J Infect Dis. 2008. PMID: 18422438
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
-
Is interferon-gamma the right marker for bacille Calmette-Guérin-induced immune protection? The missing link in our understanding of tuberculosis immunology.Clin Exp Immunol. 2012 Sep;169(3):213-9. doi: 10.1111/j.1365-2249.2012.04614.x. Clin Exp Immunol. 2012. PMID: 22861360 Free PMC article. Review.
Cited by
-
Modeling Mycobacterium tuberculosis early granuloma formation in experimental human lung tissue.Dis Model Mech. 2014 Feb;7(2):281-8. doi: 10.1242/dmm.013854. Epub 2013 Nov 7. Dis Model Mech. 2014. PMID: 24203885 Free PMC article.
-
Adenoviral vector-based strategies against infectious disease and cancer.Hum Vaccin Immunother. 2016 Aug 2;12(8):2064-2074. doi: 10.1080/21645515.2016.1165908. Epub 2016 Apr 22. Hum Vaccin Immunother. 2016. PMID: 27105067 Free PMC article. Review.
-
Advances in protein subunit vaccines against tuberculosis.Front Immunol. 2023 Aug 15;14:1238586. doi: 10.3389/fimmu.2023.1238586. eCollection 2023. Front Immunol. 2023. PMID: 37654500 Free PMC article. Review.
-
Increased B and T Cell Responses in M. bovis Bacille Calmette-Guérin Vaccinated Pigs Co-Immunized with Plasmid DNA Encoding a Prototype Tuberculosis Antigen.PLoS One. 2015 Jul 14;10(7):e0132288. doi: 10.1371/journal.pone.0132288. eCollection 2015. PLoS One. 2015. PMID: 26172261 Free PMC article.
-
Recombinant BCG: Innovations on an Old Vaccine. Scope of BCG Strains and Strategies to Improve Long-Lasting Memory.Front Immunol. 2014 Apr 7;5:152. doi: 10.3389/fimmu.2014.00152. eCollection 2014. Front Immunol. 2014. PMID: 24778634 Free PMC article. Review.
References
-
- Andersen P, Doherty TM. The success and failure of BCG: implications for a novel tuberculosis vaccine. Nat Rev. 2005;3:656–62. - PubMed
-
- Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. - PubMed
-
- Stenger S, et al. Differential effects of cytolytic T cell subsets on intracellular infection. Science. 1997;276:1684–7. - PubMed
-
- Stenger S, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

