Telomerase deficiency does not alter bleomycin-induced fibrosis in mice
- PMID: 22394286
- PMCID: PMC4046256
- DOI: 10.3109/01902148.2012.658148
Telomerase deficiency does not alter bleomycin-induced fibrosis in mice
Abstract
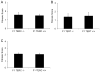
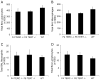
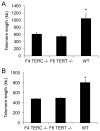
Idiopathic pulmonary fibrosis (IPF) is characterized by interstitial lung infiltrates, dyspnea, and progressive respiratory failure. Reports linking telomerase mutations to familial interstitial pneumonia (FIP) suggest that telomerase activity and telomere length maintenance are important in disease pathogenesis. To investigate the role of telomerase in lung fibrotic remodeling, intratracheal bleomycin was administered to mice deficient in telomerase reverse transcriptase (TERT) or telomerase RNA component (TERC) and to wild-type controls. TERT-deficient and TERC-deficient mice were interbred to the F6 and F4 generation, respectively, when they developed skin manifestations and infertility. Fibrosis was scored using a semiquantitative scale and total lung collagen was measured using a hydroxyprolinemicroplate assay. Telomere lengths were measured in peripheral blood leukocytes and isolated type II alveolar epithelial cells (AECs). Telomerase activity in type II AECs was measured using a real-time polymerase chain reaction (PCR)-based system. Following bleomycin, TERT-deficient and TERC-deficient mice developed an equivalent inflammatory response and similar lung fibrosis (by scoring of lung sections and total lung collagen content) compared to controls, a pattern seen in both early (F1) and later (F6 TERT and F4 TERC) generations. Telomere lengths were reduced in peripheral blood leukocytes and isolated type II AECs from F6 TERT-deficient and F4 TERC-deficient mice compared to controls. Telomerase deficiency in a murine model leads to telomere shortening, but does not predispose to enhanced bleomycin-induced lung fibrosis. Additional genetic or environmental factors may be necessary for development of fibrosis in the presence of telomerase deficiency.
Conflict of interest statement
All of the authors confirm that they have no competing or conflicting interests regarding the investigations and results outlined in this manuscript. All authors are employees of Vanderbilt University. In addition, Drs. Blackwell and Lawson are also supported by the Department of Veterans Affairs.
Figures



Similar articles
-
Genetic studies provide clues on the pathogenesis of idiopathic pulmonary fibrosis.Dis Model Mech. 2013 Jan;6(1):9-17. doi: 10.1242/dmm.010736. Dis Model Mech. 2013. PMID: 23268535 Free PMC article. Review.
-
Telomerase and telomere length in pulmonary fibrosis.Am J Respir Cell Mol Biol. 2013 Aug;49(2):260-8. doi: 10.1165/rcmb.2012-0514OC. Am J Respir Cell Mol Biol. 2013. PMID: 23526226 Free PMC article. Clinical Trial.
-
Intratracheal bleomycin causes airway remodeling and airflow obstruction in mice.Exp Lung Res. 2012 Apr;38(3):135-46. doi: 10.3109/01902148.2012.658595. Exp Lung Res. 2012. PMID: 22394287 Free PMC article.
-
KLF4 regulates TERT expression in alveolar epithelial cells in pulmonary fibrosis.Cell Death Dis. 2022 May 4;13(5):435. doi: 10.1038/s41419-022-04886-7. Cell Death Dis. 2022. PMID: 35508454 Free PMC article.
-
[A concise review of telomere and telomerase-related genetic markers in fibrotic lung diseases].Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2020 Dec 20;38(12):952-956. doi: 10.3760/cma.j.cn121094-20200305-00104. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2020. PMID: 33406566 Review. Chinese.
Cited by
-
Genetic studies provide clues on the pathogenesis of idiopathic pulmonary fibrosis.Dis Model Mech. 2013 Jan;6(1):9-17. doi: 10.1242/dmm.010736. Dis Model Mech. 2013. PMID: 23268535 Free PMC article. Review.
-
Overexpression of Telomerase Protects Human and Murine Lung Epithelial Cells from Fas- and Bleomycin-Induced Apoptosis via FLIP Upregulation.PLoS One. 2015 May 7;10(5):e0126730. doi: 10.1371/journal.pone.0126730. eCollection 2015. PLoS One. 2015. PMID: 25951185 Free PMC article.
-
Redox signaling as a therapeutic target to inhibit myofibroblast activation in degenerative fibrotic disease.Biomed Res Int. 2014;2014:131737. doi: 10.1155/2014/131737. Epub 2014 Feb 20. Biomed Res Int. 2014. PMID: 24701562 Free PMC article.
-
Telomerase reverse transcriptase ameliorates lung fibrosis by protecting alveolar epithelial cells against senescence.J Biol Chem. 2019 May 31;294(22):8861-8871. doi: 10.1074/jbc.RA118.006615. Epub 2019 Apr 18. J Biol Chem. 2019. PMID: 31000627 Free PMC article.
-
Exploring Animal Models That Resemble Idiopathic Pulmonary Fibrosis.Front Med (Lausanne). 2017 Jul 28;4:118. doi: 10.3389/fmed.2017.00118. eCollection 2017. Front Med (Lausanne). 2017. PMID: 28804709 Free PMC article. Review.
References
-
- Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE, Jr, Kondoh Y, Myers J, Muller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schunemann HJ. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. - PMC - PubMed
-
- American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–664. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL092870/HL/NHLBI NIH HHS/United States
- HL085406/HL/NHLBI NIH HHS/United States
- HL085317/HL/NHLBI NIH HHS/United States
- TL1 RR024978/RR/NCRR NIH HHS/United States
- KL2 RR024977/RR/NCRR NIH HHS/United States
- R01 HL085317/HL/NHLBI NIH HHS/United States
- HL092870/HL/NHLBI NIH HHS/United States
- T32 HL087738/HL/NHLBI NIH HHS/United States
- UL1 RR024975/RR/NCRR NIH HHS/United States
- R01 HL105479/HL/NHLBI NIH HHS/United States
- HL087738/HL/NHLBI NIH HHS/United States
- K08 HL085406/HL/NHLBI NIH HHS/United States
- HL105479/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
