Muscarinic Acetylcholine Receptor Subtypes as Potential Drug Targets for the Treatment of Schizophrenia, Drug Abuse and Parkinson's Disease
- PMID: 22389751
- PMCID: PMC3290131
- DOI: 10.1021/cn200110q
Muscarinic Acetylcholine Receptor Subtypes as Potential Drug Targets for the Treatment of Schizophrenia, Drug Abuse and Parkinson's Disease
Abstract
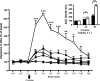
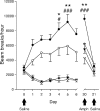
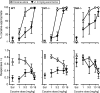
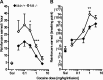
The neurotransmitter dopamine plays important roles in modulating cognitive, affective, and motor functions. Dysregulation of dopaminergic neurotransmission is thought to be involved in the pathophysiology of several psychiatric and neurological disorders, including schizophrenia, Parkinson's disease and drug abuse. Dopaminergic systems are regulated by cholinergic, especially muscarinic, input. Not surprisingly, increasing evidence implicates muscarinic acetylcholine receptor-mediated pathways as potential targets for the treatment of these disorders classically viewed as "dopamine based". There are five known muscarinic receptor subtypes (M(1) to M(5)). Due to their overlapping expression patterns and the lack of receptor subtype-specific ligands, the roles of the individual muscarinic receptors have long remained elusive. During the past decade, studies with knock-out mice lacking specific muscarinic receptor subtypes have greatly advanced our knowledge of the physiological roles of the M(1)-M(5) receptors. Recently, new ligands have been developed that can interact with allosteric sites on different muscarinic receptor subtypes, rather than the conventional (orthosteric) acetylcholine binding site. Such agents may lead to the development of novel classes of drugs useful for the treatment of psychosis, drug abuse and Parkinson's disease. The present review highlights recent studies carried out using muscarinic receptor knock-out mice and new subtype-selective allosteric ligands to assess the roles of M(1), M(4), and M(5) receptors in various central processes that are under strong dopaminergic control. The outcome of these studies opens new perspectives for the use of novel muscarinic drugs for several severe disorders of the CNS.
Figures

Similar articles
-
Physiological roles of CNS muscarinic receptors gained from knockout mice.Neuropharmacology. 2018 Jul 1;136(Pt C):411-420. doi: 10.1016/j.neuropharm.2017.09.011. Epub 2017 Sep 11. Neuropharmacology. 2018. PMID: 28911965 Free PMC article. Review.
-
Enhancement of D1 dopamine receptor-mediated locomotor stimulation in M(4) muscarinic acetylcholine receptor knockout mice.Proc Natl Acad Sci U S A. 1999 Aug 31;96(18):10483-8. doi: 10.1073/pnas.96.18.10483. Proc Natl Acad Sci U S A. 1999. PMID: 10468635 Free PMC article.
-
Novel M(1) allosteric ligands: a patent review.Expert Opin Ther Pat. 2012 Dec;22(12):1385-98. doi: 10.1517/13543776.2012.731395. Epub 2012 Oct 23. Expert Opin Ther Pat. 2012. PMID: 23092292 Review.
-
Involvement of Striatal Cholinergic Interneurons and M1 and M4 Muscarinic Receptors in Motor Symptoms of Parkinson's Disease.J Neurosci. 2016 Aug 31;36(35):9161-72. doi: 10.1523/JNEUROSCI.0873-16.2016. J Neurosci. 2016. PMID: 27581457 Free PMC article.
-
Multitargeting nature of muscarinic orthosteric agonists and antagonists.Front Physiol. 2022 Sep 6;13:974160. doi: 10.3389/fphys.2022.974160. eCollection 2022. Front Physiol. 2022. PMID: 36148314 Free PMC article. Review.
Cited by
-
G protein-coupled receptors in neurodegenerative diseases and psychiatric disorders.Signal Transduct Target Ther. 2023 May 3;8(1):177. doi: 10.1038/s41392-023-01427-2. Signal Transduct Target Ther. 2023. PMID: 37137892 Free PMC article. Review.
-
Discovery, synthesis and characterization of a highly muscarinic acetylcholine receptor (mAChR)-selective M5-orthosteric antagonist, VU0488130 (ML381): a novel molecular probe.ChemMedChem. 2014 Aug;9(8):1677-82. doi: 10.1002/cmdc.201402051. Epub 2014 Apr 1. ChemMedChem. 2014. PMID: 24692176 Free PMC article.
-
Discovery and Optimization of Potent and CNS Penetrant M5-Preferring Positive Allosteric Modulators Derived from a Novel, Chiral N-(Indanyl)piperidine Amide Scaffold.ACS Chem Neurosci. 2018 Jul 18;9(7):1572-1581. doi: 10.1021/acschemneuro.8b00126. Epub 2018 Apr 26. ACS Chem Neurosci. 2018. PMID: 29678111 Free PMC article.
-
Co-stimulation of muscarinic M1 and M4 acetylcholine receptors prevents later cocaine reinforcement in male and female mice, but not place-conditioning.Prog Neuropsychopharmacol Biol Psychiatry. 2024 Aug 30;134:111079. doi: 10.1016/j.pnpbp.2024.111079. Epub 2024 Jun 29. Prog Neuropsychopharmacol Biol Psychiatry. 2024. PMID: 38950842
-
African-specific variability in the acetylcholine muscarinic receptor M4: association with cocaine and heroin addiction.Pharmacogenomics. 2016 Jun;17(9):995-1003. doi: 10.2217/pgs-2016-0028. Epub 2016 Jun 7. Pharmacogenomics. 2016. PMID: 27269905 Free PMC article.
References
-
- Smythies J. (2005) Section I. The cholinergic system. Int. Rev. Neurobiol. 64, 1–122. - PubMed
-
- Wess J.; Eglen R. M.; Gautam D. (2007) Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat. Rev. Drug Discovery 6, 721–733. - PubMed
-
- Langmead C. J.; Watson J.; Reavill C. (2008a) Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol. Ther. 117, 232–243. - PubMed
-
- Di Chiara G.; Morelli M.; Consolo S. (1994) Modulatory functions of neurotransmitters in the striatum: ACh/dopamine/NMDA interactions. Trends Neurosci. 17, 228–233. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

