Bone marrow-derived progenitor cells augment venous remodeling in a mouse dorsal skinfold chamber model
- PMID: 22389724
- PMCID: PMC3289672
- DOI: 10.1371/journal.pone.0032815
Bone marrow-derived progenitor cells augment venous remodeling in a mouse dorsal skinfold chamber model
Abstract
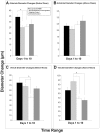
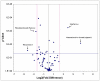
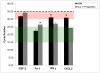
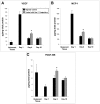
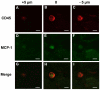
The delivery of bone marrow-derived cells (BMDCs) has been widely used to stimulate angiogenesis and arteriogenesis. We identified a progenitor-enriched subpopulation of BMDCs that is able to augment venular remodeling, a generally unexplored area in microvascular research. Two populations of BMDCs, whole bone marrow (WBM) and Lin(-)/Sca-1(+) progenitor cells, were encapsulated in sodium alginate and delivered to a mouse dorsal skinfold chamber model. Upon observation that encapsulated Sca-1(+) progenitor cells enhance venular remodeling, the cells and tissue were analyzed on structural and molecular levels. Venule walls were thickened and contained more nuclei after Sca-1(+) progenitor cell delivery. In addition, progenitors expressed mRNA transcript levels of chemokine (C-X-C motif) ligand 2 (CXCL2) and interferon gamma (IFNγ) that are over 5-fold higher compared to WBM. Tissues that received progenitors expressed significantly higher protein levels of vascular endothelial growth factor (VEGF), monocyte chemotactic protein-1 (MCP-1), and platelet derived growth factor-BB (PDGF-BB) compared to tissues that received an alginate control construct. Nine days following cell delivery, tissue from progenitor recipients contained 39% more CD45(+) leukocytes, suggesting that these cells may enhance venular remodeling through the modulation of the local immune environment. Results show that different BMDC populations elicit different microvascular responses. In this model, Sca-1(+) progenitor cell-derived CXCL2 and IFNγ may mediate venule enlargement via modulation of the local inflammatory environment.
Conflict of interest statement
Figures

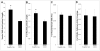


Similar articles
-
Arteriolar and venular remodeling are differentially regulated by bone marrow-derived cell-specific CX3CR1 and CCR2 expression.PLoS One. 2012;7(9):e46312. doi: 10.1371/journal.pone.0046312. Epub 2012 Sep 24. PLoS One. 2012. PMID: 23029475 Free PMC article.
-
Thrombopoietin, but not erythropoietin, directly stimulates multilineage growth of primitive murine bone marrow progenitor cells in synergy with early acting cytokines: distinct interactions with the ligands for c-kit and FLT3.Blood. 1996 Dec 15;88(12):4481-92. Blood. 1996. PMID: 8977240
-
Mouse aorta-derived mesenchymal progenitor cells contribute to and enhance the immune response of macrophage cells under inflammatory conditions.Stem Cell Res Ther. 2015 Apr 14;6(1):56. doi: 10.1186/s13287-015-0071-8. Stem Cell Res Ther. 2015. PMID: 25889992 Free PMC article.
-
CD34⁺/M-cadherin⁺ bone marrow progenitor cells promote arteriogenesis in ischemic hindlimbs of ApoE⁻/⁻ mice.PLoS One. 2011;6(6):e20673. doi: 10.1371/journal.pone.0020673. Epub 2011 Jun 3. PLoS One. 2011. PMID: 21677770 Free PMC article.
-
Combined signaling through interleukin-7 receptors and flt3 but not c-kit potently and selectively promotes B-cell commitment and differentiation from uncommitted murine bone marrow progenitor cells.Blood. 1996 Aug 15;88(4):1256-65. Blood. 1996. PMID: 8695843
Cited by
-
Dorsal skinfold chamber models in mice.GMS Interdiscip Plast Reconstr Surg DGPW. 2017 Jul 10;6:Doc10. doi: 10.3205/iprs000112. eCollection 2017. GMS Interdiscip Plast Reconstr Surg DGPW. 2017. PMID: 28706772 Free PMC article.
-
Engineering in vivo gradients of sphingosine-1-phosphate receptor ligands for localized microvascular remodeling and inflammatory cell positioning.Acta Biomater. 2014 Nov;10(11):4704-4714. doi: 10.1016/j.actbio.2014.08.007. Epub 2014 Aug 13. Acta Biomater. 2014. PMID: 25128750 Free PMC article.
-
Arteriolar and venular remodeling are differentially regulated by bone marrow-derived cell-specific CX3CR1 and CCR2 expression.PLoS One. 2012;7(9):e46312. doi: 10.1371/journal.pone.0046312. Epub 2012 Sep 24. PLoS One. 2012. PMID: 23029475 Free PMC article.
-
Hijacking the vasculature in ccRCC--co-option, remodelling and angiogenesis.Nat Rev Urol. 2013 May;10(5):300-4. doi: 10.1038/nrurol.2013.26. Epub 2013 Mar 5. Nat Rev Urol. 2013. PMID: 23459032 Review.
References
-
- Kalka C, Masuda H, Takahashi T, Gordon R, Tepper O, et al. Vascular endothelial growth factor(165) gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res. 2000;86:1198–1202. - PubMed
-
- Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, et al. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004;94:230–238. - PubMed
-
- Goodell MA, Jackson KA, Majka SM, Mi T, Wang H, et al. Stem cell plasticity in muscle and bone marrow. Ann N Y Acad Sci. 2001;938:208–218; discussion 218–220. - PubMed
-
- Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, et al. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem. 2003;89:1235–1249. - PubMed
-
- Bobis S, Jarocha D, Majka M. Mesenchymal stem cells: characteristics and clinical applications. Folia Histochem Cytobiol. 2006;44:215–230. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

