Pituitary adenylate cyclase-activating polypeptide causes tyrosine phosphorylation of the epidermal growth factor receptor in lung cancer cells
- PMID: 22389426
- PMCID: PMC3362890
- DOI: 10.1124/jpet.111.190033
Pituitary adenylate cyclase-activating polypeptide causes tyrosine phosphorylation of the epidermal growth factor receptor in lung cancer cells
Abstract
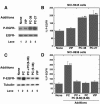
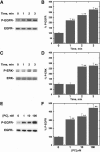
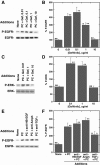
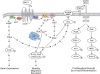
Pituitary adenylate cyclase-activating polypeptide (PACAP) is an autocrine growth factor for some lung cancer cells. The activated PACAP receptor (PAC1) causes phosphatidylinositol turnover, elevates cAMP, and increases the proliferation of lung cancer cells. PAC1 and epidermal growth factor receptor (EGFR) are present in non-small-cell lung cancer (NSCLC) cells, and the growth of NSCLC cells is inhibited by the PAC1 antagonist PACAP(6-38) and the EGFR tyrosine kinase inhibitor gefitinib. Here, the ability of PACAP to transactivate the EGFR was investigated. Western blot analysis indicated that the addition of PACAP but not the structurally related vasoactive intestinal peptide increased EGFR tyrosine phosphorylation in NCI-H838 or H345 cells. PACAP-27, in a concentration-dependent manner, increased EGFR transactivation 4-fold 2 min after addition to NCI-H838 cells. The ability of 100 nM PACAP-27 to increase EGFR or extracellular signal-regulated kinase tyrosine phosphorylation in NCI-H838 cells was inhibited by PACAP(6-38), gefitinib, 4-amino-5-(4-chlorophenyl)-7-(dimethylethyl)pyrazolo[3,4-d]pyrimidine (PP2; Src inhibitor), (R)-N4-hydroxy-N1-[(S)-2-(1H-indol-3-yl)-1-methylcarbamoyl-ethyl]-2-isobutyl-succinamide (GM6001; matrix metalloprotease inhibitor), or antibody to transforming growth factor α (TGFα). By enzyme-linked immunosorbent assay, PACAP addition to NCI-H838 cells increased TGFα secretion into conditioned media. EGFR transactivation caused by the addition of PACAP to NCI-H838 cells was inhibited by N-acetyl-cysteine (antioxidant), tiron (superoxide scavenger), diphenylene iodonium (NADPH oxidase inhibitor), or 1-[6-[[(17β)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione (U73122; phospholipase C inhibitor), but not N-[2-[[3-(4-bromophenyl)-2-propenyl]amino]ethyl]-5-isoquinolinesulfonamide (H89; protein kinase A inhibitor). PACAP addition to NCI-H838 cells significantly increased reactive oxygen species, and the increase was inhibited by tiron. The results indicate that PACAP causes transactivation of the EGFR in NSCLC cells in an oxygen-dependent manner that involves phospholipase C but not protein kinase A.
Figures

Similar articles
-
The G Protein-Coupled Receptor PAC1 Regulates Transactivation of the Receptor Tyrosine Kinase HER3.J Mol Neurosci. 2021 Aug;71(8):1589-1597. doi: 10.1007/s12031-020-01711-8. Epub 2020 Sep 22. J Mol Neurosci. 2021. PMID: 32964398 Free PMC article.
-
PYK-2 is tyrosine phosphorylated after activation of pituitary adenylate cyclase activating polypeptide receptors in lung cancer cells.J Mol Neurosci. 2012 Nov;48(3):660-6. doi: 10.1007/s12031-012-9785-6. Epub 2012 May 12. J Mol Neurosci. 2012. PMID: 22581436 Free PMC article.
-
Pituitary adenylate cyclase activating peptide receptors regulate the growth of non-small cell lung cancer cells.Cancer Res. 1995 Nov 1;55(21):4886-91. Cancer Res. 1995. PMID: 7585525 Free PMC article.
-
[Physiological significance of pituitary adenylate cyclase-activating polypeptide (PACAP) in the nervous system].Yakugaku Zasshi. 2002 Dec;122(12):1109-21. doi: 10.1248/yakushi.122.1109. Yakugaku Zasshi. 2002. PMID: 12510388 Review. Japanese.
-
[Pituitary adenylate cyclase-activating polypeptide].Ann Endocrinol (Paris). 1998 Dec;59(5):364-405. Ann Endocrinol (Paris). 1998. PMID: 9949891 Review. French.
Cited by
-
The G Protein-Coupled Receptor PAC1 Regulates Transactivation of the Receptor Tyrosine Kinase HER3.J Mol Neurosci. 2021 Aug;71(8):1589-1597. doi: 10.1007/s12031-020-01711-8. Epub 2020 Sep 22. J Mol Neurosci. 2021. PMID: 32964398 Free PMC article.
-
Targeting VIP and PACAP Receptor Signaling: New Insights into Designing Drugs for the PACAP Subfamily of Receptors.Int J Mol Sci. 2022 Jul 22;23(15):8069. doi: 10.3390/ijms23158069. Int J Mol Sci. 2022. PMID: 35897648 Free PMC article. Review.
-
Vasoactive intestinal peptide/pituitary adenylate cyclase activating polypeptide, and their receptors and cancer.Curr Opin Endocrinol Diabetes Obes. 2016 Feb;23(1):38-47. doi: 10.1097/MED.0000000000000218. Curr Opin Endocrinol Diabetes Obes. 2016. PMID: 26702849 Free PMC article. Review.
-
Influence of terminal differentiation and PACAP on the cytokine, chemokine, and growth factor secretion of mammary epithelial cells.J Mol Neurosci. 2014 Jan;52(1):28-36. doi: 10.1007/s12031-013-0193-3. Epub 2013 Dec 10. J Mol Neurosci. 2014. PMID: 24323361
-
Endothelin causes transactivation of the EGFR and HER2 in non-small cell lung cancer cells.Peptides. 2017 Apr;90:90-99. doi: 10.1016/j.peptides.2017.01.012. Epub 2017 Jan 31. Peptides. 2017. PMID: 28153500 Free PMC article.
References
-
- Arimura A. (1992) Pituitary adenylate cyclase activating polypeptide (PACAP): discovery and current status of research. Regul Pept 37:287–303 - PubMed
-
- Bhola NE, Grandis JR. (2008) Crosstalk between G-protein-coupled receptors and epidermal growth factor receptor in cancer. Front Biosci 13:1857–1865 - PubMed
-
- Buscail L, Cambillau C, Seva C, Scemama JL, De Neef P, Robberecht P, Christophe J, Susini C, Vaysse N. (1992) Stimulation of rat pancreatic tumoral AR4–2J cell proliferation by pituitary adenylate cyclase-activating peptide. Gastroenterology 103:1002–1008 - PubMed
-
- Cattaneo F, Iaccio A, Guerra G, Montagnani S, Ammendola R. (2011) NADPH-oxidase-dependent reactive oxygen species mediate EGFR transactivation by FPRL1 in WKYMVm-stimulated human lung cancer cells. Free Radic Biol Med 51:1126–1136 - PubMed
-
- Draoui M, Hida T, Jakowlew S, Birrer M, Zia F, Moody TW. (1996) PACAP stimulates c-fos mRNAs in small cell lung cancer cells. Life Sci 59:307–313 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
