Discovery of a novel Toxoplasma gondii conoid-associated protein important for parasite resistance to reactive nitrogen intermediates
- PMID: 22387554
- PMCID: PMC3320748
- DOI: 10.4049/jimmunol.1101425
Discovery of a novel Toxoplasma gondii conoid-associated protein important for parasite resistance to reactive nitrogen intermediates
Abstract
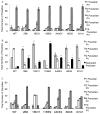
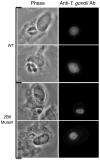
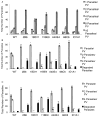
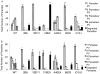
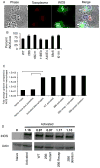
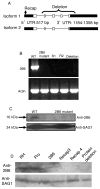
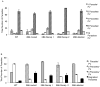
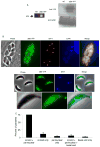
Toxoplasma gondii modifies its host cell to suppress its ability to become activated in response to IFN-γ and TNF-α and to develop intracellular antimicrobial effectors, including NO. Mechanisms used by T. gondii to modulate activation of its infected host cell likely underlie its ability to hijack monocytes and dendritic cells during infection to disseminate to the brain and CNS where it converts to bradyzoites contained in tissue cysts to establish persistent infection. To identify T. gondii genes important for resistance to the effects of host cell activation, we developed an in vitro murine macrophage infection and activation model to identify parasite insertional mutants that have a fitness defect in infected macrophages following activation but normal invasion and replication in naive macrophages. We identified 14 independent T. gondii insertional mutants out of >8000 screened that share a defect in their ability to survive macrophage activation due to macrophage production of reactive nitrogen intermediates (RNIs). These mutants have been designated counter-immune mutants. We successfully used one of these mutants to identify a T. gondii cytoplasmic and conoid-associated protein important for parasite resistance to macrophage RNIs. Deletion of the entire gene or just the region encoding the protein in wild-type parasites recapitulated the RNI-resistance defect in the counter-immune mutant, confirming the role of the protein in resistance to macrophage RNIs.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures
Similar articles
-
A patatin-like protein protects Toxoplasma gondii from degradation in activated macrophages.Mol Microbiol. 2007 Jan;63(2):482-96. doi: 10.1111/j.1365-2958.2006.05538.x. Epub 2006 Dec 11. Mol Microbiol. 2007. PMID: 17166175 Free PMC article.
-
Forward genetics screens using macrophages to identify Toxoplasma gondii genes important for resistance to IFN-γ-dependent cell autonomous immunity.J Vis Exp. 2015 Mar 12;(97):52556. doi: 10.3791/52556. J Vis Exp. 2015. PMID: 25867017 Free PMC article.
-
Hypermigration of macrophages through the concerted action of GRA effectors on NF-κB/p38 signaling and host chromatin accessibility potentiates Toxoplasma dissemination.mBio. 2024 Oct 16;15(10):e0214024. doi: 10.1128/mbio.02140-24. Epub 2024 Aug 29. mBio. 2024. PMID: 39207098 Free PMC article.
-
ROP16-Mediated Activation of STAT6 Suppresses Host Cell Reactive Oxygen Species Production, Facilitating Type III Toxoplasma gondii Growth and Survival.mBio. 2021 Mar 2;12(2):e03305-20. doi: 10.1128/mBio.03305-20. mBio. 2021. PMID: 33653884 Free PMC article.
-
Three Toxoplasma gondii Dense Granule Proteins Are Required for Induction of Lewis Rat Macrophage Pyroptosis.mBio. 2019 Jan 8;10(1):e02388-18. doi: 10.1128/mBio.02388-18. mBio. 2019. PMID: 30622189 Free PMC article.
Cited by
-
Phagocyte responses to protozoan infection and how Toxoplasma gondii meets the challenge.PLoS Pathog. 2012;8(8):e1002794. doi: 10.1371/journal.ppat.1002794. Epub 2012 Aug 2. PLoS Pathog. 2012. PMID: 22876173 Free PMC article. Review. No abstract available.
-
Molecular characterization of the conoid complex in Toxoplasma reveals its conservation in all apicomplexans, including Plasmodium species.PLoS Biol. 2021 Mar 11;19(3):e3001081. doi: 10.1371/journal.pbio.3001081. eCollection 2021 Mar. PLoS Biol. 2021. PMID: 33705380 Free PMC article.
-
An evolutionarily conserved SSNA1/DIP13 homologue is a component of both basal and apical complexes of Toxoplasma gondii.Sci Rep. 2016 Jun 21;6:27809. doi: 10.1038/srep27809. Sci Rep. 2016. PMID: 27324377 Free PMC article.
-
A Toxoplasma gondii patatin-like phospholipase contributes to host cell invasion.PLoS Pathog. 2020 Jul 6;16(7):e1008650. doi: 10.1371/journal.ppat.1008650. eCollection 2020 Jul. PLoS Pathog. 2020. PMID: 32628723 Free PMC article.
-
Targeting Toxoplasma tubules: tubulin, microtubules, and associated proteins in a human pathogen.Eukaryot Cell. 2015 Jan;14(1):2-12. doi: 10.1128/EC.00225-14. Epub 2014 Nov 7. Eukaryot Cell. 2015. PMID: 25380753 Free PMC article. Review.
References
-
- Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. - PubMed
-
- Adams LB, Hibbs JB, Jr, Taintor RR, Krahenbuhl JL. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J Immunol. 1990;144:2725–2729. - PubMed
-
- Collazo CM, Yap GS, Hieny S, Caspar P, Feng CG, Taylor GA, Sher A. The function of gamma interferon-inducible GTP-binding protein IGTP in host resistance to Toxoplasma gondii is Stat1 dependent and requires expression in both hematopoietic and nonhematopoietic cellular compartments. Infect Immun. 2002;70:6933–6939. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

