HIV-2 viral protein X (Vpx) ubiquitination is dispensable for ubiquitin ligase interaction and effects on macrophage infection
- PMID: 22386056
- PMCID: PMC3302174
- DOI: 10.1016/j.virol.2012.02.002
HIV-2 viral protein X (Vpx) ubiquitination is dispensable for ubiquitin ligase interaction and effects on macrophage infection
Abstract
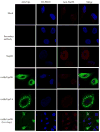
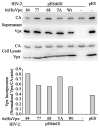
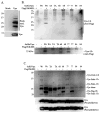
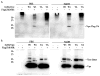
HIV-2 Vpx, a virus-associated accessory protein, is critical for infection of non-dividing myeloid cells. To understand the function of Vpx ubiquitination, interaction with an E3 ubiquitin ligase complex, and ability to overcome an inhibition of reverse transcription, we analyzed Vpx lysine mutants for their function and replication capability in macrophages. Both Wt Vpx and Vpx TA (lysine-less Vpx) localized to the cytoplasm and nucleus in HeLa cells. All HIV-2 Vpx lysine mutants were functional in virion packaging. However, ubiquitination was absent with Vpx TA and Vpx K84A mutants, indicating a lack of ubiquitin on positions K68 and K77. Mutants Vpx K68A and K77A were unable to infect macrophages due to impaired reverse transcription from loss of interaction with the ubiquitin substrate receptor, DCAF1. Even though Vpx K84A lacked ubiquitination, it bound DCAF1, and infected macrophages comparable to Wt Vpx.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Inhibition of Vpx-Mediated SAMHD1 and Vpr-Mediated Host Helicase Transcription Factor Degradation by Selective Disruption of Viral CRL4 (DCAF1) E3 Ubiquitin Ligase Assembly.J Virol. 2017 Apr 13;91(9):e00225-17. doi: 10.1128/JVI.00225-17. Print 2017 May 1. J Virol. 2017. PMID: 28202763 Free PMC article.
-
The human immunodeficiency virus type 2 Vpx protein usurps the CUL4A-DDB1 DCAF1 ubiquitin ligase to overcome a postentry block in macrophage infection.J Virol. 2009 May;83(10):4854-60. doi: 10.1128/JVI.00187-09. Epub 2009 Mar 4. J Virol. 2009. PMID: 19264781 Free PMC article.
-
HIV/simian immunodeficiency virus (SIV) accessory virulence factor Vpx loads the host cell restriction factor SAMHD1 onto the E3 ubiquitin ligase complex CRL4DCAF1.J Biol Chem. 2012 Apr 6;287(15):12550-8. doi: 10.1074/jbc.M112.340711. Epub 2012 Feb 23. J Biol Chem. 2012. PMID: 22362772 Free PMC article.
-
CRL4-DCAF1 Ubiquitin Ligase Dependent Functions of HIV Viral Protein R and Viral Protein X.Viruses. 2024 Aug 17;16(8):1313. doi: 10.3390/v16081313. Viruses. 2024. PMID: 39205287 Free PMC article. Review.
-
Lentivirus Vpr and Vpx accessory proteins usurp the cullin4-DDB1 (DCAF1) E3 ubiquitin ligase.Curr Opin Virol. 2012 Dec;2(6):755-63. doi: 10.1016/j.coviro.2012.09.010. Epub 2012 Oct 10. Curr Opin Virol. 2012. PMID: 23062609 Free PMC article. Review.
Cited by
-
Role of poly-proline motif in HIV-2 Vpx expression.Front Microbiol. 2014 Jan 28;5:24. doi: 10.3389/fmicb.2014.00024. eCollection 2014. Front Microbiol. 2014. PMID: 24478770 Free PMC article. No abstract available.
-
Incomplete Suppression of HIV-1 by SAMHD1 Permits Efficient Macrophage Infection.Pathog Immun. 2018;3(2):197-223. doi: 10.20411/pai.v3i2.263. Epub 2018 Dec 6. Pathog Immun. 2018. PMID: 30656243 Free PMC article.
-
Inhibitory Effects of HIV-2 Vpx on Replication of HIV-1.J Virol. 2018 Jun 29;92(14):e00554-18. doi: 10.1128/JVI.00554-18. Print 2018 Jul 15. J Virol. 2018. PMID: 29743354 Free PMC article.
References
-
- Angers S, Li T, Yi X, MacCoss MJ, Moon RT, Zheng N. Molecular architecture and assembly of the DDB1-CUL4A ubiquitin ligase machinery. Nature. 2006;443(7111):590–3. - PubMed
-
- Belshan M, Mahnke LA, Ratner L. Conserved amino acids of the human immunodeficiency virus type 2 Vpx nuclear localization signal are critical for nuclear targeting of the viral preintegration complex in non-dividing cells. Virology. 2006;346(1):118–26. - PubMed
-
- Belshan M, Ratner L. Identification of the nuclear localization signal of human immunodeficiency virus type 2 Vpx. Virology. 2003;311(1):7–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

