Inhibition of transforming growth factor-activated kinase 1 (TAK1) blocks and reverses epithelial to mesenchymal transition of mesothelial cells
- PMID: 22384029
- PMCID: PMC3288041
- DOI: 10.1371/journal.pone.0031492
Inhibition of transforming growth factor-activated kinase 1 (TAK1) blocks and reverses epithelial to mesenchymal transition of mesothelial cells
Abstract
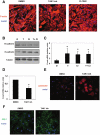
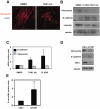
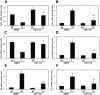
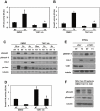
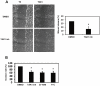
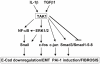
Peritoneal fibrosis is a frequent complication of peritoneal dialysis following repeated low grade inflammatory and pro-fibrotic insults. This pathological process may lead to ultrafiltration failure and eventually to the discontinuing of the therapy. Fibrosis is linked to epithelial to mesenchymal transition (EMT) of the peritoneal mesothelial cells, which acquire invasive and fibrogenic abilities. Here, we analyzed the role of the transforming growth factor-activated kinase-1 (TAK1) in the EMT of primary mesothelial cells from human peritoneum. The inhibition of TAK1 in mesenchymal-like mesothelial cells from the effluents of patients undergoing peritoneal dialysis led to the reacquisition of the apical to basolateral polarity, to increased expression of epithelial and to down-regulation of mesenchymal markers. TAK1 inhibition also resulted in decreased migratory/invasive abilities of effluent-derived mesothelial cells. Simultaneous inhibition of ERK1/2 and TAK1 pathways did not lead to an additive effect in the reacquisition of the epithelial phenotype. Inhibition of TAK1 also blocked EMT in vitro and reduced the levels of PAI-1, which is involved in fibrosis and invasion. Analysis of signalling pathways downstream of TAK1 involved in EMT induction, showed that TAK1 inhibition reduced the transcriptional activity of NF-κB and Smad3, as well as the phosphorylation of c-jun, while enhancing Smad1-5-8 activity. These results demonstrate that TAK1 is a cross-point in a network including different pro-EMT transcription factors, such as NF-κB, Snail, AP-1 and Smads. The identification of TAK1 as a main biochemical mediator of EMT and fibrosis in mesothelial cells from human peritoneum and the study of signalling pathways induced by its activity may be relevant in the design of new therapies aimed to counteract peritoneal fibrosis.
Conflict of interest statement
Figures
Similar articles
-
Curcumin suppresses epithelial-to-mesenchymal transition of peritoneal mesothelial cells (HMrSV5) through regulation of transforming growth factor-activated kinase 1 (TAK1).Cell Mol Biol Lett. 2019 May 22;24:32. doi: 10.1186/s11658-019-0157-x. eCollection 2019. Cell Mol Biol Lett. 2019. PMID: 31143210 Free PMC article.
-
p38 maintains E-cadherin expression by modulating TAK1-NF-kappa B during epithelial-to-mesenchymal transition.J Cell Sci. 2010 Dec 15;123(Pt 24):4321-31. doi: 10.1242/jcs.071647. Epub 2010 Nov 23. J Cell Sci. 2010. PMID: 21098640
-
Histone acetyltransferase inhibitor C646 reverses epithelial to mesenchymal transition of human peritoneal mesothelial cells via blocking TGF-β1/Smad3 signaling pathway in vitro.Int J Clin Exp Pathol. 2015 Mar 1;8(3):2746-54. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26045780 Free PMC article.
-
Reprogramming of Mesothelial-Mesenchymal Transition in Chronic Peritoneal Diseases by Estrogen Receptor Modulation and TGF-β1 Inhibition.Int J Mol Sci. 2020 Jun 10;21(11):4158. doi: 10.3390/ijms21114158. Int J Mol Sci. 2020. PMID: 32532126 Free PMC article. Review.
-
Mechanisms of epithelial-mesenchymal transition of peritoneal mesothelial cells during peritoneal dialysis.J Korean Med Sci. 2007 Dec;22(6):943-5. doi: 10.3346/jkms.2007.22.6.943. J Korean Med Sci. 2007. PMID: 18162703 Free PMC article. Review.
Cited by
-
Increased Expression of TGF-β Signaling Components in a Mouse Model of Fibrosis Induced by Submandibular Gland Duct Ligation.PLoS One. 2015 May 8;10(5):e0123641. doi: 10.1371/journal.pone.0123641. eCollection 2015. PLoS One. 2015. PMID: 25955532 Free PMC article.
-
Regulation of Mesothelial Cell Fate during Development and Human Diseases.Int J Mol Sci. 2022 Oct 8;23(19):11960. doi: 10.3390/ijms231911960. Int J Mol Sci. 2022. PMID: 36233262 Free PMC article. Review.
-
Impact of AMP-Activated Protein Kinase α1 Deficiency on Tissue Injury following Unilateral Ureteral Obstruction.PLoS One. 2015 Aug 18;10(8):e0135235. doi: 10.1371/journal.pone.0135235. eCollection 2015. PLoS One. 2015. PMID: 26285014 Free PMC article.
-
Curcumin suppresses epithelial-to-mesenchymal transition of peritoneal mesothelial cells (HMrSV5) through regulation of transforming growth factor-activated kinase 1 (TAK1).Cell Mol Biol Lett. 2019 May 22;24:32. doi: 10.1186/s11658-019-0157-x. eCollection 2019. Cell Mol Biol Lett. 2019. PMID: 31143210 Free PMC article.
-
Astragalus membranaceus inhibits peritoneal fibrosis via monocyte chemoattractant protein (MCP)-1 and the transforming growth factor-β1 (TGF-β1) pathway in rats submitted to peritoneal dialysis.Int J Mol Sci. 2014 Jul 22;15(7):12959-71. doi: 10.3390/ijms150712959. Int J Mol Sci. 2014. PMID: 25054320 Free PMC article.
References
-
- Aroeira LS, Aguilera A, Sanchez-Tomero JA, Bajo MA, del Peso G, et al. Epithelial to mesenchymal transition and peritoneal membrane failure in peritoneal dialysis patients: pathologic significance and potential therapeutic interventions. J Am Soc Nephrol. 2007;18:2004–2013. - PubMed
-
- Yanez-Mo M, Lara-Pezzi E, Selgas R, Ramirez-Huesca M, Dominguez-Jimenez C, et al. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N Engl J Med. 2003;348:403–413. - PubMed
-
- Zeisberg M, Yang C, Martino M, Duncan MB, Rieder F, et al. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem. 2007;282:23337–23347. - PubMed
-
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

