MicroRNA-deficient NK cells exhibit decreased survival but enhanced function
- PMID: 22379033
- PMCID: PMC3311726
- DOI: 10.4049/jimmunol.1102294
MicroRNA-deficient NK cells exhibit decreased survival but enhanced function
Abstract
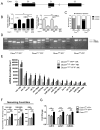
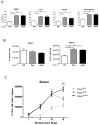
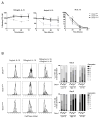
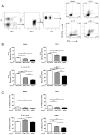
NK cells are innate immune lymphocytes important for early host defense against infectious pathogens and malignant transformation. MicroRNAs (miRNAs) are small RNA molecules that regulate a wide variety of cellular processes, typically by specific complementary targeting of the 3'UTR of mRNAs. The Dicer1 gene encodes a conserved enzyme essential for miRNA processing, and Dicer1 deficiency leads to a global defect in miRNA biogenesis. In this study, we report a mouse model of lymphocyte-restricted Dicer1 disruption to evaluate the role of Dicer1-dependent miRNAs in the development and function of NK cells. As expected, Dicer1-deficient NK cells had decreased total miRNA content. Furthermore, miRNA-deficient NK cells exhibited reduced survival and impaired maturation defined by cell surface phenotypic markers. However, Dicer1-deficient NK cells exhibited enhanced degranulation and IFN-γ production in vitro in response to cytokines, tumor target cells, and activating NK cell receptor ligation. Moreover, a similar phenotype of increased IFN-γ was evident during acute MCMV infection in vivo. miRs-15a/15b/16 were identified as abundant miRNAs in NK cells that directly target the murine IFN-γ 3'UTR, thereby providing a potential mechanism for enhanced IFN-γ production. These data suggest that the function of miRNAs in NK cell biology is complex, with an important role in NK cell development, survival, or homeostasis, while tempering peripheral NK cell activation. Further study of individual miRNAs in an NK cell specific fashion will provide insight into these complex miRNA regulatory effects in NK cell biology.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures



Similar articles
-
NKp46-mediated Dicer1 inactivation results in defective NK-cell differentiation and effector functions in mice.Eur J Immunol. 2016 Aug;46(8):1902-11. doi: 10.1002/eji.201546163. Epub 2016 Jun 8. Eur J Immunol. 2016. PMID: 27195970
-
Requirement for natural killer cell-produced interferon gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration.J Exp Med. 1995 Oct 1;182(4):1045-56. doi: 10.1084/jem.182.4.1045. J Exp Med. 1995. PMID: 7561678 Free PMC article.
-
Cytokine-Mediated Activation of NK Cells during Viral Infection.J Virol. 2015 Aug;89(15):7922-31. doi: 10.1128/JVI.00199-15. Epub 2015 May 20. J Virol. 2015. PMID: 25995253 Free PMC article.
-
microRNA management of NK-cell developmental and functional programs.Eur J Immunol. 2014 Oct;44(10):2862-8. doi: 10.1002/eji.201444798. Epub 2014 Sep 16. Eur J Immunol. 2014. PMID: 25142111 Free PMC article. Review.
-
The Role of microRNAs in NK Cell Development and Function.Cells. 2021 Aug 7;10(8):2020. doi: 10.3390/cells10082020. Cells. 2021. PMID: 34440789 Free PMC article. Review.
Cited by
-
MicroRNA-15/16 Antagonizes Myb To Control NK Cell Maturation.J Immunol. 2015 Sep 15;195(6):2806-17. doi: 10.4049/jimmunol.1500949. Epub 2015 Aug 12. J Immunol. 2015. PMID: 26268657 Free PMC article.
-
miR-146a negatively regulates NK cell functions via STAT1 signaling.Cell Mol Immunol. 2017 Aug;14(8):712-720. doi: 10.1038/cmi.2015.113. Epub 2016 Mar 21. Cell Mol Immunol. 2017. PMID: 26996068 Free PMC article.
-
NK cells and their ability to modulate T cells during virus infections.Crit Rev Immunol. 2014;34(5):359-88. doi: 10.1615/critrevimmunol.2014010604. Crit Rev Immunol. 2014. PMID: 25404045 Free PMC article. Review.
-
miRNA Regulation of NK Cells Antiviral Response in Children With Severe and/or Recurrent Herpes Simplex Virus Infections.Front Immunol. 2021 Jan 25;11:589866. doi: 10.3389/fimmu.2020.589866. eCollection 2020. Front Immunol. 2021. PMID: 33679688 Free PMC article.
-
MicroRNA-155 regulates interferon-γ production in natural killer cells via Tim-3 signalling in chronic hepatitis C virus infection.Immunology. 2015 Aug;145(4):485-97. doi: 10.1111/imm.12463. Epub 2015 Apr 21. Immunology. 2015. PMID: 25772938 Free PMC article. Clinical Trial.
References
-
- Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu Rev Immunol. 2004;22:405–29. - PubMed
-
- Di Santo JP. Natural killer cells: diversity in search of a niche. Nat Immunol. 2008;9:473–5. - PubMed
-
- Kim S, Iizuka K, Kang HSP, Dokun A, French AR, Greco S, Yokoyama WM. In vivo developmental stages in murine natural killer cell maturation. Nat Immunol. 2002;3:523–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

