PpsR, a regulator of heme and bacteriochlorophyll biosynthesis, is a heme-sensing protein
- PMID: 22378778
- PMCID: PMC3340169
- DOI: 10.1074/jbc.M112.346494
PpsR, a regulator of heme and bacteriochlorophyll biosynthesis, is a heme-sensing protein
Abstract
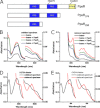
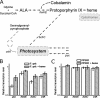
Heme-mediated regulation, presented in many biological processes, is achieved in part with proteins containing heme regulatory motif. In this study, we demonstrate that FLAG-tagged PpsR isolated from Rhodobacter sphaeroides cells contains bound heme. In vitro heme binding studies with tagless apo-PpsR show that PpsR binds heme at a near one-to-one ratio with a micromolar binding constant. Mutational and spectral assays suggest that both the second Per-Arnt-Sim (PAS) and DNA binding domains of PpsR are involved in the heme binding. Furthermore, we show that heme changes the DNA binding patterns of PpsR and induces different responses of photosystem genes expression. Thus, PpsR functions as both a redox and heme sensor to coordinate the amount of heme, bacteriochlorophyll, and photosystem apoprotein synthesis thereby providing fine tune control to avoid excess free tetrapyrrole accumulation.
Figures
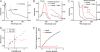
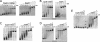


Similar articles
-
Redox and light control the heme-sensing activity of AppA.mBio. 2013 Aug 27;4(5):e00563-13. doi: 10.1128/mBio.00563-13. mBio. 2013. PMID: 23982072 Free PMC article.
-
Transcriptome analysis of the Rhodobacter sphaeroides PpsR regulon: PpsR as a master regulator of photosystem development.J Bacteriol. 2005 Mar;187(6):2148-56. doi: 10.1128/JB.187.6.2148-2156.2005. J Bacteriol. 2005. PMID: 15743963 Free PMC article.
-
Sequencing, chromosomal inactivation, and functional expression in Escherichia coli of ppsR, a gene which represses carotenoid and bacteriochlorophyll synthesis in Rhodobacter sphaeroides.J Bacteriol. 1994 May;176(10):2869-76. doi: 10.1128/jb.176.10.2869-2876.1994. J Bacteriol. 1994. PMID: 8188588 Free PMC article.
-
PpsR: a multifaceted regulator of photosynthesis gene expression in purple bacteria.Mol Microbiol. 2005 Jul;57(1):17-26. doi: 10.1111/j.1365-2958.2005.04655.x. Mol Microbiol. 2005. PMID: 15948946 Review.
-
Features of Bacteriochlorophylls Axial Ligation in the Photosynthetic Reaction Center of Purple Bacteria.Biochemistry (Mosc). 2019 Apr;84(4):370-379. doi: 10.1134/S0006297919040047. Biochemistry (Mosc). 2019. PMID: 31228928 Review.
Cited by
-
Members of the PpaA/AerR Antirepressor Family Bind Cobalamin.J Bacteriol. 2015 Aug;197(16):2694-703. doi: 10.1128/JB.00374-15. Epub 2015 Jun 8. J Bacteriol. 2015. PMID: 26055116 Free PMC article.
-
Redox Regulation of a Light-Harvesting Antenna Complex in an Anoxygenic Phototroph.mBio. 2019 Nov 26;10(6):e02838-19. doi: 10.1128/mBio.02838-19. mBio. 2019. PMID: 31772049 Free PMC article.
-
Mixotrophic growth of bacteriochlorophyll a-containing members of the OM60/NOR5 clade of marine gammaproteobacteria is carbon-starvation independent and correlates with the type of carbon source and oxygen availability.BMC Microbiol. 2013 May 24;13:117. doi: 10.1186/1471-2180-13-117. BMC Microbiol. 2013. PMID: 23705861 Free PMC article.
-
Redox and light control the heme-sensing activity of AppA.mBio. 2013 Aug 27;4(5):e00563-13. doi: 10.1128/mBio.00563-13. mBio. 2013. PMID: 23982072 Free PMC article.
-
Heme sensor proteins.J Biol Chem. 2013 May 10;288(19):13194-203. doi: 10.1074/jbc.R112.422642. Epub 2013 Mar 28. J Biol Chem. 2013. PMID: 23539616 Free PMC article. Review.
References
-
- Marks G. S. (1969) Heme and Chlorophyll, pp. 6–25, D. Van Nostrand Company, Princeton, NJ
-
- Boon E. M., Huang S. H., Marletta M. A. (2005) A molecular basis for NO selectivity in soluble guanylate cyclase. Nat. Chem. Biol. 1, 53–59 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

