The zinc transporter Zip14 influences c-Met phosphorylation and hepatocyte proliferation during liver regeneration in mice
- PMID: 22374166
- PMCID: PMC3635537
- DOI: 10.1053/j.gastro.2012.02.046
The zinc transporter Zip14 influences c-Met phosphorylation and hepatocyte proliferation during liver regeneration in mice
Abstract
Background & aims: Zinc homeostasis in cells is maintained through tight regulation of zinc influx, efflux, and distribution to intracellular organelles by zinc transporters. The Zrt-Irt-like protein (ZIP) transporters facilitate zinc influx to the cytosol. Expression of the ZIP family member Zip14 can be induced by inflammatory cytokines, which also initiate liver regeneration. Hepatocyte proliferation is required for liver regeneration. Zinc regulates cell proliferation, tissue growth, and many mitogenic signaling pathways; we investigated its role in hepatocytes.
Methods: Wild-type and Zip14(-/-) mice that underwent partial hepatectomy (70% of liver removed) were used as models of liver regeneration. We also analyzed AML12 hepatocytes that overexpressed Zip14. Proliferation was assessed with proliferating cell nuclear antigen, CD1, and Ki67 markers and along with assays of zinc content was related to protein tyrosine phosphatase 1B (PTP1B) and extracellular signal-regulated kinase 1/2 signaling.
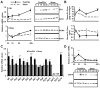
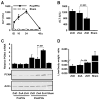
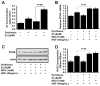
Results: Zip14 was up-regulated and hepatic zinc content increased during liver regeneration. Increased hepatic zinc inhibited activity of the phosphatase PTP1B and increased phosphorylation of c-Met, which promoted hepatocyte proliferation. AML12 cells that overexpressed Zip14 increased in zinc content and proliferation; PTP1B was inhibited and phosphorylation of c-Met increased. The increases in hepatic levels of zinc and hepatocyte proliferation that occurred following partial hepatectomy were not observed in Zip14(-/-) mice.
Conclusions: The transporter Zip14 mediates hepatic uptake of zinc during liver regeneration and for hepatocyte proliferation. These findings indicate that zinc transporter activity regulates liver tissue growth by sequestering zinc. Reagents that regulate ZIP14 activity might be developed as therapeutics to promote liver regeneration in patients with chronic liver disease.
Copyright © 2012 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest
The authors disclose no conflicts.
Figures
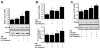


Similar articles
-
Hepatic ZIP14-mediated zinc transport is required for adaptation to endoplasmic reticulum stress.Proc Natl Acad Sci U S A. 2017 Jul 18;114(29):E5805-E5814. doi: 10.1073/pnas.1704012114. Epub 2017 Jul 3. Proc Natl Acad Sci U S A. 2017. PMID: 28673968 Free PMC article.
-
Zinc transporter ZIP14 functions in hepatic zinc, iron and glucose homeostasis during the innate immune response (endotoxemia).PLoS One. 2012;7(10):e48679. doi: 10.1371/journal.pone.0048679. Epub 2012 Oct 24. PLoS One. 2012. PMID: 23110240 Free PMC article.
-
Protein Tyrosine Phosphatase 1B (PTP1B) deficiency accelerates hepatic regeneration in mice.Am J Pathol. 2011 Apr;178(4):1591-604. doi: 10.1016/j.ajpath.2010.12.020. Epub 2011 Mar 4. Am J Pathol. 2011. PMID: 21406170 Free PMC article.
-
The Multiple Faces of the Metal Transporter ZIP14 (SLC39A14).J Nutr. 2018 Feb 1;148(2):174-184. doi: 10.1093/jn/nxx041. J Nutr. 2018. PMID: 29490098 Free PMC article. Review.
-
Influence of ZIP14 (slc39A14) on intestinal zinc processing and barrier function.Am J Physiol Gastrointest Liver Physiol. 2015 Feb 1;308(3):G171-8. doi: 10.1152/ajpgi.00021.2014. Epub 2014 Nov 26. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 25428902 Free PMC article. Review.
Cited by
-
The Zinc Sensing Receptor, ZnR/GPR39, in Health and Disease.Int J Mol Sci. 2018 Feb 1;19(2):439. doi: 10.3390/ijms19020439. Int J Mol Sci. 2018. PMID: 29389900 Free PMC article. Review.
-
Clioquinol synergistically augments rescue by zinc supplementation in a mouse model of acrodermatitis enteropathica.PLoS One. 2013 Aug 28;8(8):e72543. doi: 10.1371/journal.pone.0072543. eCollection 2013. PLoS One. 2013. PMID: 24015258 Free PMC article.
-
Gene expression of the zinc transporter ZIP14 (SLC39a14) is affected by weight loss and metabolic status and associates with PPARγ in human adipose tissue and 3T3-L1 pre-adipocytes.BMC Obes. 2015 Nov 24;2:46. doi: 10.1186/s40608-015-0076-y. eCollection 2015. BMC Obes. 2015. PMID: 26623077 Free PMC article.
-
Absence of Slc39a14/Zip14 in mouse pancreatic beta cells results in hyperinsulinemia.Am J Physiol Endocrinol Metab. 2024 Jan 1;326(1):E92-E105. doi: 10.1152/ajpendo.00117.2023. Epub 2023 Nov 29. Am J Physiol Endocrinol Metab. 2024. PMID: 38019082 Free PMC article.
-
Expression analysis of zinc transporters in resting and stimulated human peripheral blood mononuclear cells.Biomed Rep. 2014 Mar;2(2):217-222. doi: 10.3892/br.2014.219. Epub 2014 Jan 13. Biomed Rep. 2014. PMID: 24649099 Free PMC article.
References
-
- Clavien PA, Oberkofler CE, Raptis DA, et al. What is critical for liver surgery and partial liver transplantation: size or quality? Hepatology. 2010;52:715–729. - PubMed
-
- Schuppan D, Popov Y. Rationale and targets for antifibrotic therapies. Gastroenterol Clin Biol. 2009;33:949–957. - PubMed
-
- Weismann K, Christensen E, Dreyer V. Zinc supplementation in alcoholic cirrhosis. A double-blind clinical trial. Acta Med Scand. 1979;205:361–366. - PubMed
-
- Murakami Y, Koyabu T, Kawashima A, et al. Zinc supplementation prevents the increase of transaminase in chronic hepatitis C patients during combination therapy with pegylated interferon alpha-2b and ribavirin. J Nutr Sci Vitaminol (Tokyo) 2007;53:213–221. - PubMed
-
- Takagi H, Nagamine T, Abe T, et al. Zinc supplementation enhances the response to interferon therapy in patients with chronic hepatitis C. J Viral Hepat. 2001;8:367–337. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

