β-N-Acetylglucosamine (O-GlcNAc) is a novel regulator of mitosis-specific phosphorylations on histone H3
- PMID: 22371497
- PMCID: PMC3320971
- DOI: 10.1074/jbc.M111.315804
β-N-Acetylglucosamine (O-GlcNAc) is a novel regulator of mitosis-specific phosphorylations on histone H3
Abstract
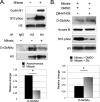
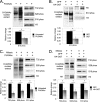
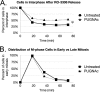
O-Linked β-N-acetylglucosamine, or O-GlcNAc, is a dynamic post-translational modification that cycles on and off serine and threonine residues of nucleocytoplasmic proteins. The O-GlcNAc modification shares a complex relationship with phosphorylation, as both modifications are capable of mutually inhibiting the occupation of each other on the same or nearby amino acid residue. In addition to diabetes, cancer, and neurodegenerative diseases, O-GlcNAc appears to play a significant role in cell growth and cell cycle progression, although the precise mechanisms are still not well understood. A recent study also found that all four core nucleosomal histones (H2A, H2B, H3, and H4) are modified with O-GlcNAc, although no specific sites on H3 were reported. Here, we describe that histone H3, a protein highly phosphorylated during mitosis, is modified with O-GlcNAc. Several biochemical assays were used to validate that H3 is modified with O-GlcNAc. Mass spectrometry analysis identified threonine 32 as a novel O-GlcNAc site. O-GlcNAc was detected at higher levels on H3 during interphase than mitosis, which inversely correlated with phosphorylation. Furthermore, increased O-GlcNAcylation was observed to reduce mitosis-specific phosphorylation at serine 10, serine 28, and threonine 32. Finally, inhibiting OGA, the enzyme responsible for removing O-GlcNAc, hindered the transition from G2 to M phase of the cell cycle, displaying a phenotype similar to preventing mitosis-specific phosphorylation on H3. Taken together, these data indicate that O-GlcNAcylation regulates mitosis-specific phosphorylations on H3, providing a mechanistic switch that orchestrates the G2-M transition of the cell cycle.
Figures



Similar articles
-
Modification of histones by sugar β-N-acetylglucosamine (GlcNAc) occurs on multiple residues, including histone H3 serine 10, and is cell cycle-regulated.J Biol Chem. 2011 Oct 28;286(43):37483-95. doi: 10.1074/jbc.M111.284885. Epub 2011 Sep 6. J Biol Chem. 2011. PMID: 21896475 Free PMC article.
-
Beta-N-acetylglucosamine (O-GlcNAc) is part of the histone code.Proc Natl Acad Sci U S A. 2010 Nov 16;107(46):19915-20. doi: 10.1073/pnas.1009023107. Epub 2010 Nov 2. Proc Natl Acad Sci U S A. 2010. PMID: 21045127 Free PMC article.
-
O-Linked N-Acetylglucosamine Transiently Elevates in HeLa Cells during Mitosis.Molecules. 2018 May 26;23(6):1275. doi: 10.3390/molecules23061275. Molecules. 2018. PMID: 29861440 Free PMC article.
-
Functional significance of O-GlcNAc modification in regulating neuronal properties.Pharmacol Res. 2018 Mar;129:295-307. doi: 10.1016/j.phrs.2017.12.006. Epub 2017 Dec 6. Pharmacol Res. 2018. PMID: 29223644 Review.
-
The sweet side of the cell cycle.Biochem Soc Trans. 2017 Apr 15;45(2):313-322. doi: 10.1042/BST20160145. Biochem Soc Trans. 2017. PMID: 28408472 Free PMC article. Review.
Cited by
-
Interplay between epigenetics and metabolism in oncogenesis: mechanisms and therapeutic approaches.Oncogene. 2017 Jun 15;36(24):3359-3374. doi: 10.1038/onc.2016.485. Epub 2017 Jan 16. Oncogene. 2017. PMID: 28092669 Free PMC article. Review.
-
Overview of the Assays to Probe O-Linked β-N-Acetylglucosamine Transferase Binding and Activity.Molecules. 2021 Feb 16;26(4):1037. doi: 10.3390/molecules26041037. Molecules. 2021. PMID: 33669256 Free PMC article. Review.
-
Interplay between Metabolism and Epigenetics: A Nuclear Adaptation to Environmental Changes.Mol Cell. 2016 Jun 2;62(5):695-711. doi: 10.1016/j.molcel.2016.05.029. Mol Cell. 2016. PMID: 27259202 Free PMC article. Review.
-
O-GlcNAcylation: the sweet side of epigenetics.Epigenetics Chromatin. 2023 Dec 14;16(1):49. doi: 10.1186/s13072-023-00523-5. Epigenetics Chromatin. 2023. PMID: 38093337 Free PMC article. Review.
-
H3K4me3 remodeling induced acquired resistance through O-GlcNAc transferase.Drug Resist Updat. 2023 Nov;71:100993. doi: 10.1016/j.drup.2023.100993. Epub 2023 Aug 10. Drug Resist Updat. 2023. PMID: 37639774 Free PMC article.
References
-
- Hart G. W., Housley M. P., Slawson C. (2007) Cycling of O-linked β-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 446, 1017–1022 - PubMed
-
- Slawson C., Hart G. W. (2003) Dynamic interplay between O-GlcNAc and O-phosphate: the sweet side of protein regulation. Curr. Opin. Struct. Biol. 13, 631–636 - PubMed
-
- Hu P., Shimoji S., Hart G. W. (2010) Site-specific interplay between O-GlcNAcylation and phosphorylation in cellular regulation. FEBS Lett. 584, 2526–2538 - PubMed
-
- Kreppel L. K., Blomberg M. A., Hart G. W. (1997) Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J. Biol. Chem. 272, 9308–9315 - PubMed
-
- Gao Y., Wells L., Comer F. I., Parker G. J., Hart G. W. (2001) Dynamic O-glycosylation of nuclear and cytosolic proteins: cloning and characterization of a neutral, cytosolic β-N-acetylglucosaminidase from human brain. J. Biol. Chem. 276, 9838–9845 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

