MTBP suppresses cell migration and filopodia formation by inhibiting ACTN4
- PMID: 22370640
- PMCID: PMC3742333
- DOI: 10.1038/onc.2012.69
MTBP suppresses cell migration and filopodia formation by inhibiting ACTN4
Abstract
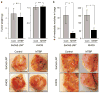
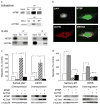
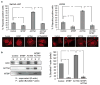
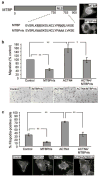
Murine double minute (MDM2) binding protein (MTBP) has been implicated in cancer progression. Here, we demonstrate one mechanism by which MTBP inhibits cancer metastasis. Overexpression of MTBP in human osteosarcoma cell lines lacking wild-type p53 did not alter primary tumor growth in mice, but significantly inhibited metastases. MTBP downregulation increased the migratory potential of MDM2(-/-)p53(-/-) mouse embryonic fibroblasts, suggesting that MTBP inhibited cell migration independently of the Mdm2-p53 pathway. Co-immunoprecipitation and mass spectrometric analysis identified alpha-actinin-4 (ACTN4) as an MTBP-interacting protein. Endogenous MTBP interacted with and partially colocalized with ACTN4. MTBP overexpression inhibited cell migration and filopodia formation mediated by ACTN4. Increased cell migration by MTBP downregulation was inhibited by concomitant downregulation of ACTN4. MTBP also inhibited ACTN4-mediated F-actin bundling. We furthermore demonstrated that nuclear localization of MTBP was dispensable for inhibiting ACTN4-mediated cell migration and filopodia formation. Thus, MTBP suppresses cell migration, at least partially, by inhibiting ACTN4 function. Our study not only provides a mechanism of metastasis suppression by MTBP, but also suggests MTBP as a potential biomarker for cancer progression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
MDM2 binding protein, a novel metastasis suppressor.Cancer Metastasis Rev. 2012 Dec;31(3-4):633-40. doi: 10.1007/s10555-012-9364-x. Cancer Metastasis Rev. 2012. PMID: 22684342 Review.
-
MTBP inhibits migration and metastasis of hepatocellular carcinoma.Clin Exp Metastasis. 2015 Apr;32(4):301-11. doi: 10.1007/s10585-015-9706-5. Epub 2015 Mar 11. Clin Exp Metastasis. 2015. PMID: 25759210 Free PMC article.
-
Mtbp haploinsufficiency in mice increases tumor metastasis.Oncogene. 2008 Mar 20;27(13):1813-20. doi: 10.1038/sj.onc.1210827. Epub 2007 Oct 1. Oncogene. 2008. PMID: 17906694
-
Long Non-Coding RNA CRYBG3 Promotes Lung Cancer Metastasis via Activating the eEF1A1/MDM2/MTBP Axis.Int J Mol Sci. 2021 Mar 22;22(6):3211. doi: 10.3390/ijms22063211. Int J Mol Sci. 2021. PMID: 33809929 Free PMC article.
-
Role of ACTN4 in Tumorigenesis, Metastasis, and EMT.Cells. 2019 Nov 13;8(11):1427. doi: 10.3390/cells8111427. Cells. 2019. PMID: 31766144 Free PMC article. Review.
Cited by
-
Comparative iTRAQ proteomics revealed proteins associated with horn development in yak.Proteome Sci. 2018 Jul 24;16:14. doi: 10.1186/s12953-018-0141-9. eCollection 2018. Proteome Sci. 2018. PMID: 30061793 Free PMC article.
-
Hyper expression of MTBP may be an adverse signal for the survival of some malignant tumors: A data-based analysis and clinical observation.Medicine (Baltimore). 2018 Aug;97(35):e12021. doi: 10.1097/MD.0000000000012021. Medicine (Baltimore). 2018. PMID: 30170409 Free PMC article.
-
MTBP inhibits the Erk1/2-Elk-1 signaling in hepatocellular carcinoma.Oncotarget. 2018 Apr 20;9(30):21429-21443. doi: 10.18632/oncotarget.25117. eCollection 2018 Apr 20. Oncotarget. 2018. PMID: 29765550 Free PMC article.
-
The sodium channel β1 subunit mediates outgrowth of neurite-like processes on breast cancer cells and promotes tumour growth and metastasis.Int J Cancer. 2014 Nov 15;135(10):2338-51. doi: 10.1002/ijc.28890. Epub 2014 Apr 26. Int J Cancer. 2014. PMID: 24729314 Free PMC article.
-
Genetic analysis of the molecular regulation of electric fields-guided glia migration.Sci Rep. 2020 Oct 8;10(1):16821. doi: 10.1038/s41598-020-74085-x. Sci Rep. 2020. PMID: 33033380 Free PMC article.
References
-
- Mina LA, Sledge GW., Jr Rethinking the metastatic cascade as a therapeutic target. Nat Rev Clin Oncol. 2011;8(6):325–32. Epub 2011/04/20. - PubMed
-
- Chen X, Xu Z, Wang Y. Recent advances in breast cancer metastasis suppressor 1. Int J Biol Markers. 2011;26(1):1–8. - PubMed
-
- Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Curr Opin Cell Biol. 2005;17(5):559–64. - PubMed
-
- Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol. 2008;9(6):446–54. - PubMed
-
- Lindberg U, Karlsson R, Lassing I, Schutt CE, Hoglund AS. The microfilament system and malignancy. Semin Cancer Biol. 2008;18(1):2–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

