The Roles of Mitogen-Activated Protein Kinase Pathways in TGF-β-Induced Epithelial-Mesenchymal Transition
- PMID: 22363839
- PMCID: PMC3272823
- DOI: 10.1155/2012/289243
The Roles of Mitogen-Activated Protein Kinase Pathways in TGF-β-Induced Epithelial-Mesenchymal Transition
Abstract
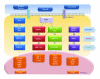
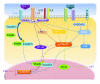
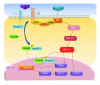
The mitogen-activated protein kinase (MAPK) pathway allows cells to interpret external signals and respond appropriately, especially during the epithelial-mesenchymal transition (EMT). EMT is an important process during embryonic development, fibrosis, and tumor progression in which epithelial cells acquire mesenchymal, fibroblast-like properties and show reduced intercellular adhesion and increased motility. TGF-β signaling is the first pathway to be described as an inducer of EMT, and its relationship with the Smad family is already well characterized. Studies of four members of the MAPK family in different biological systems have shown that the MAPK and TGF-β signaling pathways interact with each other and have a synergistic effect on the secretion of additional growth factors and cytokines that in turn promote EMT. In this paper, we present background on the regulation and function of MAPKs and their cascades, highlight the mechanisms of MAPK crosstalk with TGF-β signaling, and discuss the roles of MAPKs in EMT.
Figures
Similar articles
-
Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells.J Am Soc Nephrol. 2005 Mar;16(3):667-75. doi: 10.1681/ASN.2004050425. Epub 2005 Jan 26. J Am Soc Nephrol. 2005. PMID: 15677311
-
Role of Smad2/3 and p38 MAP kinase in TGF-β1-induced epithelial-mesenchymal transition of pulmonary epithelial cells.J Cell Physiol. 2011 May;226(5):1248-54. doi: 10.1002/jcp.22448. J Cell Physiol. 2011. PMID: 20945383 Free PMC article.
-
Total flavone of Abelmoschus manihot suppresses epithelial-mesenchymal transition via interfering transforming growth factor-β1 signaling in Crohn's disease intestinal fibrosis.World J Gastroenterol. 2018 Aug 14;24(30):3414-3425. doi: 10.3748/wjg.v24.i30.3414. World J Gastroenterol. 2018. PMID: 30122880 Free PMC article.
-
TGF-β signaling and epithelial-mesenchymal transition in cancer progression.Curr Opin Oncol. 2013 Jan;25(1):76-84. doi: 10.1097/CCO.0b013e32835b6371. Curr Opin Oncol. 2013. PMID: 23197193 Review.
-
Epithelial-mesenchymal transition is regulated at post-transcriptional levels by transforming growth factor-β signaling during tumor progression.Cancer Sci. 2015 May;106(5):481-8. doi: 10.1111/cas.12630. Epub 2015 Mar 9. Cancer Sci. 2015. PMID: 25664423 Free PMC article. Review.
Cited by
-
MAPKs in the early steps of senescence implemEMTation.Front Cell Dev Biol. 2023 Mar 16;11:1083401. doi: 10.3389/fcell.2023.1083401. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37009481 Free PMC article.
-
Trehalose ameliorates peritoneal fibrosis by promoting Snail degradation and inhibiting mesothelial-to-mesenchymal transition in mesothelial cells.Sci Rep. 2020 Aug 31;10(1):14292. doi: 10.1038/s41598-020-71230-4. Sci Rep. 2020. PMID: 32868830 Free PMC article.
-
Role of TGF-β in Skin Chronic Wounds: A Keratinocyte Perspective.Cells. 2020 Jan 28;9(2):306. doi: 10.3390/cells9020306. Cells. 2020. PMID: 32012802 Free PMC article. Review.
-
Anti-migration and anti-invasion effects of LY-290181 on breast cancer cell lines through the inhibition of Twist1.BMB Rep. 2023 Jul;56(7):410-415. doi: 10.5483/BMBRep.2023-0011. BMB Rep. 2023. PMID: 37357535 Free PMC article.
-
Stimulation of Chondrogenesis in a Developmental Model of Endochondral Bone Formation by Pulsed Electromagnetic Fields.Int J Mol Sci. 2023 Feb 7;24(4):3275. doi: 10.3390/ijms24043275. Int J Mol Sci. 2023. PMID: 36834690 Free PMC article. Review.
References
-
- Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410(6824):37–40. - PubMed
-
- Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nature Reviews Cancer. 2009;9(8):537–549. - PubMed
-
- Lee SJ, Zhou T, Goldsmith EJ. Crystallization of MAP kinases. Methods. 2006;40(3):224–233. - PubMed
LinkOut - more resources
Full Text Sources

