Ghrelin indirectly activates hypophysiotropic CRF neurons in rodents
- PMID: 22363652
- PMCID: PMC3282735
- DOI: 10.1371/journal.pone.0031462
Ghrelin indirectly activates hypophysiotropic CRF neurons in rodents
Abstract
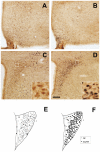
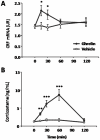
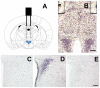
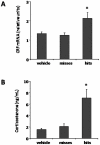
Ghrelin is a stomach-derived hormone that regulates food intake and neuroendocrine function by acting on its receptor, GHSR (Growth Hormone Secretagogue Receptor). Recent evidence indicates that a key function of ghrelin is to signal stress to the brain. It has been suggested that one of the potential stress-related ghrelin targets is the CRF (Corticotropin-Releasing Factor)-producing neurons of the hypothalamic paraventricular nucleus, which secrete the CRF neuropeptide into the median eminence and activate the hypothalamic-pituitary-adrenal axis. However, the neural circuits that mediate the ghrelin-induced activation of this neuroendocrine axis are mostly uncharacterized. In the current study, we characterized in vivo the mechanism by which ghrelin activates the hypophysiotropic CRF neurons in mice. We found that peripheral or intra-cerebro-ventricular administration of ghrelin strongly activates c-fos--a marker of cellular activation--in CRF-producing neurons. Also, ghrelin activates CRF gene expression in the paraventricular nucleus of the hypothalamus and the hypothalamic-pituitary-adrenal axis at peripheral level. Ghrelin administration directly into the paraventricular nucleus of the hypothalamus also induces c-fos within the CRF-producing neurons and the hypothalamic-pituitary-adrenal axis, without any significant effect on the food intake. Interestingly, dual-label immunohistochemical analysis and ghrelin binding studies failed to show GHSR expression in CRF neurons. Thus, we conclude that ghrelin activates hypophysiotropic CRF neurons, albeit indirectly.
Conflict of interest statement
Figures



Similar articles
-
Ghrelin activates hypophysiotropic corticotropin-releasing factor neurons independently of the arcuate nucleus.Psychoneuroendocrinology. 2016 May;67:27-39. doi: 10.1016/j.psyneuen.2016.01.027. Epub 2016 Feb 1. Psychoneuroendocrinology. 2016. PMID: 26874559 Free PMC article.
-
GHSR controls food deprivation-induced activation of CRF neurons of the hypothalamic paraventricular nucleus in a LEAP2-dependent manner.Cell Mol Life Sci. 2022 May 4;79(5):277. doi: 10.1007/s00018-022-04302-5. Cell Mol Life Sci. 2022. PMID: 35504998 Free PMC article.
-
Divergence in the expression of molecular markers of neuronal activation in the parvocellular paraventricular nucleus of the hypothalamus evoked by alcohol administration via different routes.J Neurosci. 1998 Jun 1;18(11):4344-52. doi: 10.1523/JNEUROSCI.18-11-04344.1998. J Neurosci. 1998. PMID: 9592111 Free PMC article.
-
Regulatory mechanisms underlying corticotropin-releasing factor gene expression in the hypothalamus.Endocr J. 2009;56(3):335-44. doi: 10.1507/endocrj.k09e-075. Epub 2009 Apr 7. Endocr J. 2009. PMID: 19352056 Review.
-
Role of hypothalamic corticotropin-releasing factor in mediating alcohol-induced activation of the rat hypothalamic-pituitary-adrenal axis.Front Neuroendocrinol. 2014 Apr;35(2):221-33. doi: 10.1016/j.yfrne.2013.10.005. Epub 2013 Nov 7. Front Neuroendocrinol. 2014. PMID: 24211830 Review.
Cited by
-
Circulating ghrelin changes as a biomarker of the stress response and craving in abstinent smokers.Pharmacol Biochem Behav. 2022 Jul;218:173423. doi: 10.1016/j.pbb.2022.173423. Epub 2022 Jun 21. Pharmacol Biochem Behav. 2022. PMID: 35750154 Free PMC article.
-
Divergent neuronal circuitries underlying acute orexigenic effects of peripheral or central ghrelin: critical role of brain accessibility.J Neuroendocrinol. 2014 Aug;26(8):542-54. doi: 10.1111/jne.12168. J Neuroendocrinol. 2014. PMID: 24888783 Free PMC article.
-
Constitutive and ghrelin-dependent GHSR1a activation impairs CaV2.1 and CaV2.2 currents in hypothalamic neurons.J Gen Physiol. 2015 Sep;146(3):205-19. doi: 10.1085/jgp.201511383. Epub 2015 Aug 17. J Gen Physiol. 2015. PMID: 26283199 Free PMC article.
-
Ghrelin activates hypophysiotropic corticotropin-releasing factor neurons independently of the arcuate nucleus.Psychoneuroendocrinology. 2016 May;67:27-39. doi: 10.1016/j.psyneuen.2016.01.027. Epub 2016 Feb 1. Psychoneuroendocrinology. 2016. PMID: 26874559 Free PMC article.
-
Neonatal overfeeding disrupts pituitary ghrelin signalling in female rats long-term; Implications for the stress response.PLoS One. 2017 Mar 10;12(3):e0173498. doi: 10.1371/journal.pone.0173498. eCollection 2017. PLoS One. 2017. PMID: 28282447 Free PMC article.
References
-
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. - PubMed
-
- Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. - PubMed
-
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. - PubMed
-
- Broglio F, Arvat E, Benso A, Gottero C, Muccioli G, et al. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab. 2001;86:5083–5086. - PubMed
-
- Patterson ZR, Ducharme R, Anisman H, Abizaid A. Altered metabolic and neurochemical responses to chronic unpredictable stressors in ghrelin receptor-deficient mice. Eur J Neurosci. 2010;32:632–639. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

