α1Proteinase inhibitor regulates CD4+ lymphocyte levels and is rate limiting in HIV-1 disease
- PMID: 22363634
- PMCID: PMC3281957
- DOI: 10.1371/journal.pone.0031383
α1Proteinase inhibitor regulates CD4+ lymphocyte levels and is rate limiting in HIV-1 disease
Abstract
Background: The regulation of adult stem cell migration through human hematopoietic tissue involves the chemokine CXCL12 (SDF-1) and its receptor CXCR4 (CD184). In addition, human leukocyte elastase (HLE) plays a key role. When HLE is located on the cell surface (HLE(CS)), it acts not as a proteinase, but as a receptor for α(1)proteinase inhibitor (α(1)PI, α(1)antitrypsin, SerpinA1). Binding of α(1)PI to HLE(CS) forms a motogenic complex. We previously demonstrated that α(1)PI deficiency attends HIV-1 disease and that α(1)PI augmentation produces increased numbers of immunocompetent circulating CD4(+) lymphocytes. Herein we investigated the mechanism underlying the α(1)PI deficiency that attends HIV-1 infection.
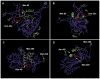
Methods and findings: Active α(1)PI in HIV-1 subjects (median 17 µM, n = 35) was significantly below normal (median 36 µM, p<0.001, n = 30). In HIV-1 uninfected subjects, CD4(+) lymphocytes were correlated with the combined factors α(1)PI, HLE(CS) (+) lymphocytes, and CXCR4(+) lymphocytes (r(2) = 0.91, p<0.001, n = 30), but not CXCL12. In contrast, in HIV-1 subjects with >220 CD4 cells/µl, CD4(+) lymphocytes were correlated solely with active α(1)PI (r(2) = 0.93, p<0.0001, n = 26). The monoclonal anti-HIV-1 gp120 antibody 3F5 present in HIV-1 patient blood is shown to bind and inactivate human α(1)PI. Chimpanzee α(1)PI differs from human α(1)PI by a single amino acid within the 3F5-binding epitope. Unlike human α(1)PI, chimpanzee α(1)PI did not bind 3F5 or become depleted following HIV-1 challenge, consistent with the normal CD4(+) lymphocyte levels and benign syndrome of HIV-1 infected chimpanzees. The presence of IgG-α(1)PI immune complexes correlated with decreased CD4(+) lymphocytes in HIV-1 subjects.
Conclusions: This report identifies an autoimmune component of HIV-1 disease that can be overcome therapeutically. Importantly, results identify an achievable vaccine modification with the novel objective to protect against AIDS as opposed to the current objective to protect against HIV-1 infection.
Conflict of interest statement
Figures

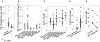
Similar articles
-
The α-test: rapid cell-free CD4 enumeration using whole saliva.J Vis Exp. 2012 May 16;(63):e3999. doi: 10.3791/3999. J Vis Exp. 2012. PMID: 22644001 Free PMC article.
-
Cocrystal Structures of Antibody N60-i3 and Antibody JR4 in Complex with gp120 Define More Cluster A Epitopes Involved in Effective Antibody-Dependent Effector Function against HIV-1.J Virol. 2015 Sep;89(17):8840-54. doi: 10.1128/JVI.01232-15. Epub 2015 Jun 17. J Virol. 2015. PMID: 26085162 Free PMC article.
-
Cross-Linking of a CD4-Mimetic Miniprotein with HIV-1 Env gp140 Alters Kinetics and Specificities of Antibody Responses against HIV-1 Env in Macaques.J Virol. 2017 Sep 12;91(19):e00401-17. doi: 10.1128/JVI.00401-17. Print 2017 Oct 1. J Virol. 2017. PMID: 28490585 Free PMC article.
-
Development of Immature CD4+CD8+T Cells Into Mature CD4+ T Cells Requires Alpha-1 Antitrypsin as Observed by Treatment in HIV-1 Infected and Uninfected Controls.Front Cell Dev Biol. 2019 Nov 21;7:278. doi: 10.3389/fcell.2019.00278. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31824943 Free PMC article.
-
The role of complement and gp120-specific antibodies in virus lysis and CD4+ T cell depletion in HIV-1-infected patients.Microb Pathog. 1998 Nov;25(5):253-66. doi: 10.1006/mpat.1998.0233. Microb Pathog. 1998. PMID: 9878454
Cited by
-
Lipoproteins in Negative Feedback with Alpha-1 Antitrypsin.Methods Mol Biol. 2024;2750:167-174. doi: 10.1007/978-1-0716-3605-3_15. Methods Mol Biol. 2024. PMID: 38108976
-
Acute-phase protein α1-anti-trypsin: diverting injurious innate and adaptive immune responses from non-authentic threats.Clin Exp Immunol. 2015 Feb;179(2):161-72. doi: 10.1111/cei.12476. Clin Exp Immunol. 2015. PMID: 25351931 Free PMC article. Review.
-
Alphataxin, a Small-Molecule Drug That Elevates Tumor-Infiltrating CD4+ T Cells, in Combination With Anti-PD-1 Therapy, Suppresses Murine Renal Cancer and Metastasis.Front Oncol. 2021 Nov 25;11:739080. doi: 10.3389/fonc.2021.739080. eCollection 2021. Front Oncol. 2021. PMID: 34900690 Free PMC article.
-
Alphataxin, an Orally Available Small Molecule, Decreases LDL Levels in Mice as a Surrogate for the LDL-Lowering Activity of Alpha-1 Antitrypsin in Humans.Front Pharmacol. 2021 Jun 9;12:695971. doi: 10.3389/fphar.2021.695971. eCollection 2021. Front Pharmacol. 2021. PMID: 34177602 Free PMC article.
-
The α-test: rapid cell-free CD4 enumeration using whole saliva.J Vis Exp. 2012 May 16;(63):e3999. doi: 10.3791/3999. J Vis Exp. 2012. PMID: 22644001 Free PMC article.
References
-
- Yahata T, Yumino S, Seng Y, Miyatake H, Uno T, et al. Clonal analysis of thymus-repopulating cells presents direct evidence for self-renewal division of human hematopoietic stem cells. Blood. 2006;108:2446–2454. - PubMed
-
- Lapidot T, Petit I. Current understanding of stem cell mobilization: The roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30:973–981. - PubMed
-
- Tavor S, Petit I, Porozov S, Goichberg P, Avigdor A, et al. Motility, proliferation, and egress to the circulation of human AML cells are elastase dependent in NOD/SCID chimeric mice. Blood. 2005;106:2120–2127. - PubMed
-
- Cepinskas G, Sandig M, Kvietys PR. PAF-induced elastase-dependent neutrophil transendothelial migration is associated with the mobilization of elastase to the neutrophil surface and localization to the migrating front. J Cell Science. 1999;112:1937–1945. - PubMed
-
- Horwitz M, Benson KF, Person RE, Aprikyan AG, Dale DC. Mutations in ELA2, encoding neutrophil elastase, define a 21-day clock in cyclic haematopoiesis. Nat Genet. 1999;23:433436. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

