First-line cetuximab plus capecitabine in elderly patients with advanced colorectal cancer: clinical outcome and subgroup analysis according to KRAS status from a Spanish TTD Group Study
- PMID: 22363067
- PMCID: PMC3316919
- DOI: 10.1634/theoncologist.2011-0406
First-line cetuximab plus capecitabine in elderly patients with advanced colorectal cancer: clinical outcome and subgroup analysis according to KRAS status from a Spanish TTD Group Study
Abstract
Single-agent cetuximab is safe and active in elderly patients with advanced colorectal cancer (CRC). A cetuximab-capecitabine combination has not previously been tested in elderly patients with advanced CRC.
Material and methods: Sixty-six patients with advanced CRC were treated with cetuximab as a 400 mg/m2 i.v. infusion followed by 250 mg/m2 i.v. weekly plus capecitabine at a dose of 1,250 mg/m2 every 12 hours. After the inclusion of 27 patients, the protocol was amended for safety reasons, reducing the dose of capecitabine to 1,000 mg/m2 every 12 hours. Thirty-nine additional patients were treated with the reduced dose of capecitabine.
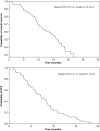
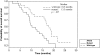
Results: The overall response rate was 31.8%. KRAS status was determined in 58 patients (88%). Fourteen of 29 patients with wild-type KRAS tumors responded (48.3%; 95% confidence interval [CI], 29.4%-67.5%), compared with six of 29 patients with mutant KRAS tumors (20.7%; 95% CI, 8.0%-39.7%). The median progression-free survival (PFS) interval was 7.1 months. The median PFS interval for patients whose tumors were wild-type KRAS was significantly longer than for those with mutant KRAS tumors (8.4 months versus 6.0 months; p = .024). The high incidence of severe paronychia (29.6%) declined (7.7%) after capecitabine dose adjustment.
Conclusions: Cetuximab plus capecitabine at a dose of 1,000 mg/m2 every 12 hours may be an alternative to more aggressive regimens in elderly patients with advanced wild-type KRAS CRC.
Conflict of interest statement
Figures

Similar articles
-
Cetuximab plus capecitabine and irinotecan compared with cetuximab plus capecitabine and oxaliplatin as first-line treatment for patients with metastatic colorectal cancer: AIO KRK-0104--a randomized trial of the German AIO CRC study group.J Clin Oncol. 2011 Mar 10;29(8):1050-8. doi: 10.1200/JCO.2010.31.1936. Epub 2011 Feb 7. J Clin Oncol. 2011. PMID: 21300933 Clinical Trial.
-
Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial.Lancet. 2011 Jun 18;377(9783):2103-14. doi: 10.1016/S0140-6736(11)60613-2. Epub 2011 Jun 5. Lancet. 2011. PMID: 21641636 Free PMC article. Clinical Trial.
-
Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer.N Engl J Med. 2009 Feb 5;360(6):563-72. doi: 10.1056/NEJMoa0808268. N Engl J Med. 2009. PMID: 19196673 Clinical Trial.
-
FOLFOX plus anti-epidermal growth factor receptor (EGFR) monoclonal antibody (mAb) is an effective first-line treatment for patients with RAS-wild left-sided metastatic colorectal cancer: A meta-analysis.Medicine (Baltimore). 2018 Mar;97(10):e0097. doi: 10.1097/MD.0000000000010097. Medicine (Baltimore). 2018. PMID: 29517682 Free PMC article. Review.
-
Cetuximab in the treatment of patients with colorectal cancer.Expert Opin Biol Ther. 2011 Jul;11(7):937-49. doi: 10.1517/14712598.2011.582464. Epub 2011 May 11. Expert Opin Biol Ther. 2011. PMID: 21557708 Review.
Cited by
-
The place of targeted agents in the treatment of elderly patients with metastatic colorectal cancer.Cancers (Basel). 2015 Mar 13;7(1):439-49. doi: 10.3390/cancers7010439. Cancers (Basel). 2015. PMID: 25782007 Free PMC article. Review.
-
Should capecitabine replace 5-fluorouracil in the first-line treatment of metastatic colorectal cancer?World J Gastroenterol. 2014 May 28;20(20):6092-101. doi: 10.3748/wjg.v20.i20.6092. World J Gastroenterol. 2014. PMID: 24876731 Free PMC article. Review.
-
Chemotherapy for colorectal cancer in the elderly.World J Gastroenterol. 2015 May 7;21(17):5158-66. doi: 10.3748/wjg.v21.i17.5158. World J Gastroenterol. 2015. PMID: 25954089 Free PMC article. Review.
-
Treatment of colorectal cancer in older patients.Nat Rev Gastroenterol Hepatol. 2012 Dec;9(12):716-25. doi: 10.1038/nrgastro.2012.196. Epub 2012 Oct 9. Nat Rev Gastroenterol Hepatol. 2012. PMID: 23045000 Review.
-
An Update on the Role of Anti-EGFR in the Treatment of Older Patients with Metastatic Colorectal Cancer.J Clin Med. 2022 Nov 30;11(23):7108. doi: 10.3390/jcm11237108. J Clin Med. 2022. PMID: 36498683 Free PMC article. Review.
References
-
- Gatta G, Faivre J, Capocaccia R, et al. The EUROCARE Working Group. Survival of colorectal cancer patients in Europe during period 1978–1989. Eur J Cancer. 1998;34:2176–2183. - PubMed
-
- Fentiman IS. Are the elderly receiving appropriate treatment for cancer? Ann Oncol. 1996;7:657–658. - PubMed
-
- Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. - PubMed
-
- Feliu J, Gonzàlez Barón M, Espinosa E, et al. Uracil and tegafur modulated with leucovorin: An effective regimen with low toxicity for the treatment of colorectal carcinoma in the elderly. Oncopaz Cooperative Group. Cancer. 1997;79:1884–1889. - PubMed
-
- Feliu J, Mel JR, Camps C, et al. Raltitrexed in the treatment of elderly patients with advanced colorectal cancer: An active and low toxicity regimen. Eur J Cancer. 2002;38:1204–1211. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

