HIV/simian immunodeficiency virus (SIV) accessory virulence factor Vpx loads the host cell restriction factor SAMHD1 onto the E3 ubiquitin ligase complex CRL4DCAF1
- PMID: 22362772
- PMCID: PMC3321004
- DOI: 10.1074/jbc.M112.340711
HIV/simian immunodeficiency virus (SIV) accessory virulence factor Vpx loads the host cell restriction factor SAMHD1 onto the E3 ubiquitin ligase complex CRL4DCAF1
Abstract
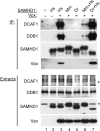
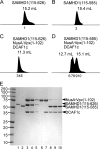
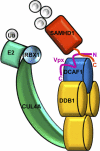
The sterile alpha motif and HD domain-containing protein-1 (SAMHD1) inhibits infection of myeloid cells by human and related primate immunodeficiency viruses (HIV and SIV). This potent inhibition is counteracted by the Vpx accessory virulence factor of HIV-2/SIVsm viruses, which targets SAMHD1 for proteasome-dependent degradation, by reprogramming cellular CRL4(DCAF1) E3 ubiquitin ligase. However, the precise mechanism of Vpx-dependent recruitment of human SAMHD1 onto the ligase, and the molecular interfaces on the respective molecules have not been defined. Here, we show that human SAMHD1 is recruited to the CRL4(DCAF1-Vpx) E3 ubiquitin ligase complex by interacting with the DCAF1 substrate receptor subunit in a Vpx-dependent manner. No stable association is detectable with DCAF1 alone. The SAMHD1 determinant for the interaction is a short peptide located distal to the SAMHD1 catalytic domain and requires the presence of Vpx for stable engagement. This peptide is sufficient to confer Vpx-dependent recruitment to CRL4(DCAF1) and ubiquitination when fused to heterologous proteins. The precise amino acid sequence of the peptide diverges among SAMHD1 proteins from different vertebrate species, explaining selective down-regulation of human SAMHD1 levels by Vpx. Critical amino acid residues of SAMHD1 and Vpx involved in the DCAF1-Vpx-SAMDH1 interaction were identified by mutagenesis. Our findings show that the N terminus of Vpx, bound to DCAF1, recruits SAMHD1 via its C terminus to CRL4, in a species-specific manner for proteasomal degradation.
Figures



Similar articles
-
Inhibition of Vpx-Mediated SAMHD1 and Vpr-Mediated Host Helicase Transcription Factor Degradation by Selective Disruption of Viral CRL4 (DCAF1) E3 Ubiquitin Ligase Assembly.J Virol. 2017 Apr 13;91(9):e00225-17. doi: 10.1128/JVI.00225-17. Print 2017 May 1. J Virol. 2017. PMID: 28202763 Free PMC article.
-
HIV-2 and SIVmac accessory virulence factor Vpx down-regulates SAMHD1 enzyme catalysis prior to proteasome-dependent degradation.J Biol Chem. 2013 Jun 28;288(26):19116-26. doi: 10.1074/jbc.M113.469007. Epub 2013 May 15. J Biol Chem. 2013. PMID: 23677995 Free PMC article.
-
Structural Basis of Clade-specific Engagement of SAMHD1 (Sterile α Motif and Histidine/Aspartate-containing Protein 1) Restriction Factors by Lentiviral Viral Protein X (Vpx) Virulence Factors.J Biol Chem. 2015 Jul 17;290(29):17935-17945. doi: 10.1074/jbc.M115.665513. Epub 2015 Jun 4. J Biol Chem. 2015. PMID: 26045556 Free PMC article.
-
Lentivirus Vpr and Vpx accessory proteins usurp the cullin4-DDB1 (DCAF1) E3 ubiquitin ligase.Curr Opin Virol. 2012 Dec;2(6):755-63. doi: 10.1016/j.coviro.2012.09.010. Epub 2012 Oct 10. Curr Opin Virol. 2012. PMID: 23062609 Free PMC article. Review.
-
CRL4-DCAF1 Ubiquitin Ligase Dependent Functions of HIV Viral Protein R and Viral Protein X.Viruses. 2024 Aug 17;16(8):1313. doi: 10.3390/v16081313. Viruses. 2024. PMID: 39205287 Free PMC article. Review.
Cited by
-
Viral evasion mechanisms of early antiviral responses involving regulation of ubiquitin pathways.Trends Microbiol. 2013 Aug;21(8):421-9. doi: 10.1016/j.tim.2013.06.006. Epub 2013 Jul 11. Trends Microbiol. 2013. PMID: 23850008 Free PMC article. Review.
-
The Vpx lentiviral accessory protein targets SAMHD1 for degradation in the nucleus.J Virol. 2012 Dec;86(23):12552-60. doi: 10.1128/JVI.01657-12. Epub 2012 Sep 12. J Virol. 2012. PMID: 22973040 Free PMC article.
-
SAMHD1-Dependent and -Independent Functions of HIV-2/SIV Vpx Protein.Front Microbiol. 2012 Aug 10;3:297. doi: 10.3389/fmicb.2012.00297. eCollection 2012. Front Microbiol. 2012. PMID: 22908011 Free PMC article.
-
The retroviral restriction ability of SAMHD1, but not its deoxynucleotide triphosphohydrolase activity, is regulated by phosphorylation.Cell Host Microbe. 2013 Apr 17;13(4):441-51. doi: 10.1016/j.chom.2013.03.005. Cell Host Microbe. 2013. PMID: 23601106 Free PMC article.
-
SAMHD1: Recurring roles in cell cycle, viral restriction, cancer, and innate immunity.Autoimmunity. 2018 May;51(3):96-110. doi: 10.1080/08916934.2018.1454912. Epub 2018 Mar 27. Autoimmunity. 2018. PMID: 29583030 Free PMC article. Review.
References
-
- Steinman R. M., Hemmi H. (2006) Dendritic cells: translating innate to adaptive immunity. Curr. Top Microbiol. Immunol. 311, 17–58 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

