Dysregulated TLR3-dependent signaling and innate immune activation in superoxide-deficient macrophages from nonobese diabetic mice
- PMID: 22361747
- PMCID: PMC3711256
- DOI: 10.1016/j.freeradbiomed.2012.01.027
Dysregulated TLR3-dependent signaling and innate immune activation in superoxide-deficient macrophages from nonobese diabetic mice
Abstract
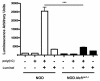
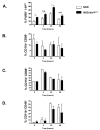
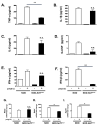
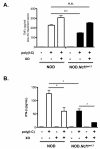
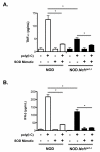
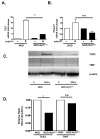
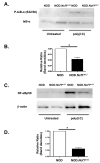
In type 1 diabetes (T1D), reactive oxygen species (ROS) and proinflammatory cytokines produced by macrophages and other innate immune cells destroy pancreatic β cells while promoting autoreactive T cell maturation. Superoxide-deficient nonobese diabetic mice (NOD.Ncf1(m1J)) are resistant to spontaneous diabetes, revealing the integral role of ROS signaling in T1D. Here, we evaluate the innate immune activation state of bone marrow-derived macrophages (BM-Mϕ) from NOD and NOD.Ncf1(m1J) mice after poly(I:C)-induced Toll-like receptor 3 (TLR3) signaling. We show that ROS synthesis is required for efficient activation of the NF-κB signaling pathway and concomitant expression of TLR3 and the cognate adaptor molecule, TRIF. Poly(I:C)-stimulated NOD.Ncf1(m1J) BM-Mϕ exhibited a 2- and 10-fold decrease in TNF-α and IFN-β proinflammatory cytokine synthesis, respectively, in contrast to NOD BM-Mϕ. Optimal expression of IFN-α/β is not solely dependent on superoxide synthesis, but requires p47(phox) to function in a NOX-independent manner to mediate type I interferon synthesis. Interestingly, MHC-II I-A(g7) expression necessary for CD4 T cell activation is increased 2-fold relative to NOD, implicating a role for superoxide in I-A(g7) downregulation. These findings suggest that defective innate immune-pattern-recognition receptor activation and subsequent decrease in TNF-α and IFN-β proinflammatory cytokine synthesis necessary for autoreactive T cell maturation may contribute to the T1D protection observed in NOD.Ncf1(m1J) mice.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
Comment in
-
Radical innate regulation of autoimmune diabetes.Free Radic Biol Med. 2012 May 1;52(9):1698-9. doi: 10.1016/j.freeradbiomed.2012.02.024. Epub 2012 Feb 26. Free Radic Biol Med. 2012. PMID: 22373596 Free PMC article. No abstract available.
Similar articles
-
The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis.Ann N Y Acad Sci. 2013 Apr;1281(1):16-35. doi: 10.1111/j.1749-6632.2012.06826.x. Epub 2013 Jan 16. Ann N Y Acad Sci. 2013. PMID: 23323860 Free PMC article. Review.
-
Loss of NADPH oxidase-derived superoxide skews macrophage phenotypes to delay type 1 diabetes.Diabetes. 2015 Mar;64(3):937-46. doi: 10.2337/db14-0929. Epub 2014 Oct 6. Diabetes. 2015. PMID: 25288672 Free PMC article.
-
Loss of NOX-Derived Superoxide Exacerbates Diabetogenic CD4 T-Cell Effector Responses in Type 1 Diabetes.Diabetes. 2015 Dec;64(12):4171-83. doi: 10.2337/db15-0546. Epub 2015 Aug 12. Diabetes. 2015. PMID: 26269022 Free PMC article.
-
Superoxide production by macrophages and T cells is critical for the induction of autoreactivity and type 1 diabetes.Diabetes. 2011 Aug;60(8):2144-51. doi: 10.2337/db10-1222. Epub 2011 Jun 29. Diabetes. 2011. PMID: 21715554 Free PMC article.
-
Mechanisms and pathways of innate immune activation and regulation in health and cancer.Hum Vaccin Immunother. 2014;10(11):3270-85. doi: 10.4161/21645515.2014.979640. Hum Vaccin Immunother. 2014. PMID: 25625930 Free PMC article. Review.
Cited by
-
Redox-Dependent Inflammation in Islet Transplantation Rejection.Front Endocrinol (Lausanne). 2018 Apr 23;9:175. doi: 10.3389/fendo.2018.00175. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29740396 Free PMC article. Review.
-
Redox-Sensitive Innate Immune Pathways During Macrophage Activation in Type 1 Diabetes.Antioxid Redox Signal. 2018 Nov 10;29(14):1373-1398. doi: 10.1089/ars.2017.7243. Epub 2017 Nov 27. Antioxid Redox Signal. 2018. PMID: 29037052 Free PMC article. Review.
-
The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis.Ann N Y Acad Sci. 2013 Apr;1281(1):16-35. doi: 10.1111/j.1749-6632.2012.06826.x. Epub 2013 Jan 16. Ann N Y Acad Sci. 2013. PMID: 23323860 Free PMC article. Review.
-
NADPH Oxidase-Derived Superoxide Provides a Third Signal for CD4 T Cell Effector Responses.J Immunol. 2016 Sep 1;197(5):1733-42. doi: 10.4049/jimmunol.1502581. Epub 2016 Jul 29. J Immunol. 2016. PMID: 27474077 Free PMC article.
-
Sumoylation modulates oxidative stress relevant to the viability and functionality of pancreatic beta cells.Am J Transl Res. 2014 Jul 18;6(4):353-60. eCollection 2014. Am J Transl Res. 2014. PMID: 25075252 Free PMC article. Review.
References
-
- D’Autreaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. - PubMed
-
- Medzhitov R, Janeway CA., Jr. Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9:4–9. - PubMed
-
- Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol. 1999;162:3256–3262. - PubMed
-
- Tse HM, Milton MJ, Schreiner S, Profozich JL, Trucco M, Piganelli JD. Disruption of innate-mediated proinflammatory cytokine and reactive oxygen species third signal leads to antigen-specific hyporesponsiveness. J Immunol. 2007;178:908–917. - PubMed
-
- Pape KA, Khoruts A, Mondino A, Jenkins MK. Inflammatory cytokines enhance the in vivo clonal expansion and differentiation of antigen-activated CD4+ T cells. J Immunol. 1997;159:591–598. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

