Brain-derived neurotrophic factor (BDNF) reverses the effects of rapid eye movement sleep deprivation (REMSD) on developmentally regulated, long-term potentiation (LTP) in visual cortex slices
- PMID: 22361363
- PMCID: PMC3307368
- DOI: 10.1016/j.neulet.2012.02.012
Brain-derived neurotrophic factor (BDNF) reverses the effects of rapid eye movement sleep deprivation (REMSD) on developmentally regulated, long-term potentiation (LTP) in visual cortex slices
Abstract
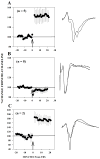
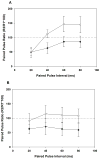
Work in this laboratory demonstrated a role for rapid eye movement sleep (REMS) in critical period (CP), postnatal days (P) 17-30, synaptic plasticity in visual cortex. Studies in adolescent rats showed that REMS deprivation (REMSD) reinitiates a developmentally regulated form of synaptic plasticity that otherwise is observed only in CP animals. Subsequent work added that REMSD affects inhibitory mechanisms that are thought to be involved in terminating the CP. Neurotrophins are implicated in the synaptic plasticity that underlies CP maturation and also final closure of the CP in visual cortex. Expression of brain-derived neurotrophic factor (BDNF) is dependent upon neuronal activity, and REMSD may block BDNF expression. We propose that REMS contributes to the maturation of visual cortex through regulation of BDNF expression and consequent, downstream increase in cortical inhibitory tone. In this study, osmotic minipumps delivered BDNF into visual cortex on one side of brain. The opposite hemisphere was not implanted and served as an internal control. We tested the hypothesis that BDNF is blocked by REMSD in late-adolescent rats and investigated whether replacing BDNF prevents induction of LTPWM-III by theta burst stimulation (TBS). We also assessed relative inhibitory tone in visual cortex with paired-pulse stimulation (PPS) in animals that were similarly REMSD- and BDNF-infused. After REMSD, both hemispheres were prepared in parallel for in vitro synaptic plasticity studies (LTPWM-III or PPS). In visual cortex of REMSD rats on the side receiving BDNF infusions (8 of 8 animals), TBS consistently failed to induce LTPWM-III. In contrast, LTPWM-III was obtained (5 of 5 animals) in the matched, non-infused hemisphere, as expected in rats of this age. REMSD animals that were unilaterally infused with saline produced LTPWM-III in both hemispheres. PPS studies in another group of REMSD animals that were unilaterally BDNF-infused displayed age-appropriate inhibition of the second response on the BDNF-infused side (5/5), whereas on the non-infused side facilitation was observed (3/3). Intracortical infusion of BDNF in REMSD adolescent rats appears to restore neurochemical processes necessary for termination of the CP for developmentally regulated synaptic plasticity in visual cortex. The results suggest that REMSD blocks BDNF expression and also maturation of inhibitory processes in adolescent visual cortex. These data support REMS' function in brain development.
Copyright © 2012 Elsevier Ireland Ltd. All rights reserved.
Figures
Similar articles
-
Rapid eye movement sleep deprivation in post-critical period, adolescent rats alters the balance between inhibitory and excitatory mechanisms in visual cortex.Neurosci Lett. 2006 Jan 30;393(2-3):131-5. doi: 10.1016/j.neulet.2005.09.051. Epub 2005 Oct 19. Neurosci Lett. 2006. PMID: 16236445
-
Rapid eye movement sleep deprivation revives a form of developmentally regulated synaptic plasticity in the visual cortex of post-critical period rats.Neurosci Lett. 2006 Jan 2;391(3):96-101. doi: 10.1016/j.neulet.2005.08.044. Epub 2005 Sep 9. Neurosci Lett. 2006. PMID: 16154270
-
Rapid eye movement sleep deprivation modifies expression of long-term potentiation in visual cortex of immature rats.Neuroscience. 2002;110(3):431-43. doi: 10.1016/s0306-4522(01)00589-9. Neuroscience. 2002. PMID: 11906784
-
BDNF mechanisms in late LTP formation: A synthesis and breakdown.Neuropharmacology. 2014 Jan;76 Pt C:664-76. doi: 10.1016/j.neuropharm.2013.06.024. Epub 2013 Jul 2. Neuropharmacology. 2014. PMID: 23831365 Review.
-
Comparison of plasticity in vivo and in vitro in the developing visual cortex of normal and protein kinase A RIbeta-deficient mice.J Neurosci. 1998 Mar 15;18(6):2108-17. doi: 10.1523/JNEUROSCI.18-06-02108.1998. J Neurosci. 1998. PMID: 9482797 Free PMC article. Review.
Cited by
-
Sleep and synaptic plasticity in the developing and adult brain.Curr Top Behav Neurosci. 2015;25:123-49. doi: 10.1007/7854_2014_305. Curr Top Behav Neurosci. 2015. PMID: 24671703 Free PMC article. Review.
-
In vivo organ specific drug delivery with implantable peristaltic pumps.Sci Rep. 2016 May 17;6:26251. doi: 10.1038/srep26251. Sci Rep. 2016. PMID: 27185292 Free PMC article.
-
Sleep and Early Cortical Development.Curr Sleep Med Rep. 2015 Mar;1(1):64-73. doi: 10.1007/s40675-014-0002-8. Epub 2015 Feb 3. Curr Sleep Med Rep. 2015. PMID: 26807347 Free PMC article.
-
Chronic Paroxetine Treatment Prevents the Emergence of Abnormal Electroencephalogram Oscillations in Huntington's Disease Mice.Neurotherapeutics. 2017 Oct;14(4):1120-1133. doi: 10.1007/s13311-017-0546-7. Neurotherapeutics. 2017. PMID: 28653279 Free PMC article.
-
The Ontogenesis of Mammalian Sleep: Form and Function.Curr Sleep Med Rep. 2020 Dec;6(4):267-279. doi: 10.1007/s40675-020-00190-y. Epub 2020 Nov 13. Curr Sleep Med Rep. 2020. PMID: 33816063 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

