Reactive oxygen production induced by the gut microbiota: pharmacotherapeutic implications
- PMID: 22360484
- PMCID: PMC4269156
- DOI: 10.2174/092986712799828283
Reactive oxygen production induced by the gut microbiota: pharmacotherapeutic implications
Abstract
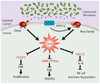
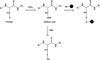
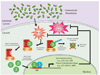
The resident prokaryotic microbiota of the mammalian intestine influences diverse homeostatic functions, including regulation of cellular growth, maintenance of barrier function, and modulation of immune responses. However, it is unknown how commensal prokaryotic organisms mechanistically influence eukaryotic signaling networks. Recent data has demonstrated that gut epithelia contacted by enteric commensal bacteria rapidly generate reactive oxygen species (ROS). While the induced generation of ROS via stimulation of formyl peptide receptors is a cardinal feature of the cellular response of phagocytes to pathogenic or commensal bacteria, evidence is accumulating that ROS are also similarly elicited in other cell types, including intestinal epithelia, in response to microbial signals. Additionally, ROS have been shown to serve as critical second messengers in multiple signal transduction pathways stimulated by proinflammatory cytokines and growth factors. This physiologically-generated ROS is known to participate in cellular signaling via the rapid and transient oxidative inactivation of a defined class of sensor proteins bearing oxidant-sensitive thiol groups. These proteins include tyrosine phosphatases that serve as regulators of MAP kinase pathways, cytoskeletal dynamics, as well as components involved in control of ubiquitination-mediated NF-κB activation. Consistently, microbial-elicited ROS has been shown to mediate increased cellular proliferation and motility and to modulate innate immune signaling. These results demonstrate how enteric microbiota influence regulatory networks of the mammalian intestinal epithelia. We hypothesize that many of the known effects of the normal microbiota on intestinal physiology, and potential beneficial effects of candidate probiotic bacteria, may be at least partially mediated by this ROS-dependent mechanism.
Figures
Similar articles
-
Redox signaling mediated by the gut microbiota.Free Radic Res. 2013 Nov;47(11):950-7. doi: 10.3109/10715762.2013.833331. Epub 2013 Oct 4. Free Radic Res. 2013. PMID: 23937589 Free PMC article. Review.
-
Redox signaling mediated by the gut microbiota.Free Radic Biol Med. 2017 Apr;105:41-47. doi: 10.1016/j.freeradbiomed.2016.10.495. Epub 2016 Oct 29. Free Radic Biol Med. 2017. PMID: 27989756 Review.
-
Commensal bacteria modulate cullin-dependent signaling via generation of reactive oxygen species.EMBO J. 2007 Oct 31;26(21):4457-66. doi: 10.1038/sj.emboj.7601867. Epub 2007 Oct 4. EMBO J. 2007. PMID: 17914462 Free PMC article.
-
Enteric commensal bacteria induce extracellular signal-regulated kinase pathway signaling via formyl peptide receptor-dependent redox modulation of dual specific phosphatase 3.J Biol Chem. 2011 Nov 4;286(44):38448-38455. doi: 10.1074/jbc.M111.268938. Epub 2011 Sep 15. J Biol Chem. 2011. PMID: 21921027 Free PMC article.
-
The bacterial fermentation product butyrate influences epithelial signaling via reactive oxygen species-mediated changes in cullin-1 neddylation.J Immunol. 2009 Jan 1;182(1):538-46. doi: 10.4049/jimmunol.182.1.538. J Immunol. 2009. PMID: 19109186 Free PMC article.
Cited by
-
Pan-Genome Analysis Reveals Functional Divergences in Gut-Restricted Gilliamella and Snodgrassella.Bioengineering (Basel). 2022 Oct 12;9(10):544. doi: 10.3390/bioengineering9100544. Bioengineering (Basel). 2022. PMID: 36290512 Free PMC article.
-
Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: a systematic review.Gut Microbes. 2020 Nov 9;12(1):1801944. doi: 10.1080/19490976.2020.1801944. Gut Microbes. 2020. PMID: 32795116 Free PMC article.
-
Gut microbiota in ischemic stroke: Where we stand and challenges ahead.Front Nutr. 2022 Dec 1;9:1008514. doi: 10.3389/fnut.2022.1008514. eCollection 2022. Front Nutr. 2022. PMID: 36532541 Free PMC article. Review.
-
Insect anal droplets contain diverse proteins related to gut homeostasis.BMC Genomics. 2018 Oct 30;19(1):784. doi: 10.1186/s12864-018-5182-z. BMC Genomics. 2018. PMID: 30376807 Free PMC article.
-
Commensal microbiota-induced redox signaling activates proliferative signals in the intestinal stem cell microenvironment.Development. 2019 Feb 1;146(3):dev171520. doi: 10.1242/dev.171520. Development. 2019. PMID: 30658986 Free PMC article.
References
-
- Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Jian M, Zhou Y, Li Y, Zhang X, Qin N, Yang H, Wang J, Brunak S, Dore J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, Bork P, Ehrlich SD. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. - PMC - PubMed
-
- Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292(5519):1115–1118. - PubMed
-
- Xu J, Mahowald MA, Ley RE, Lozupone CA, Hamady M, Martens EC, Henrissat B, Coutinho PM, Minx P, Latreille P, Cordum H, Van Brunt A, Kim K, Fulton RS, Fulton LA, Clifton SW, Wilson RK, Knight RD, Gordon JI. Evolution of symbiotic bacteria in the distal human intestine. PLoS Biol. 2007;5(7):e156. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

