Dyrk kinases regulate phosphorylation of doublecortin, cytoskeletal organization, and neuronal morphology
- PMID: 22359282
- PMCID: PMC3556588
- DOI: 10.1002/cm.21021
Dyrk kinases regulate phosphorylation of doublecortin, cytoskeletal organization, and neuronal morphology
Abstract
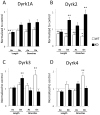
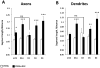
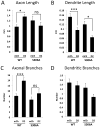
In a neuronal overexpression screen focused on kinases and phosphatases, one "hit" was the dual specificity tyrosine phosphorylation-regulated kinase (Dyrk4), which increased the number of dendritic branches in hippocampal neurons. Overexpression of various Dyrk family members in primary neurons significantly changed neuronal morphology. Dyrk1A decreased axon growth, Dyrk3 and Dyrk4 increased dendritic branching, and Dyrk2 decreased both axon and dendrite growth and branching. Kinase-deficient mutants revealed that most of these effects depend on kinase activity. Because doublecortin (DCX), a microtubule-binding protein, regulates cytoskeletal dynamics and neuronal morphogenesis, we investigated the possibility that DCX is a target of Dyrks. We found that overexpression of Dyrk2 and Dyrk3, but not Dyrk1A or Dyrk4, can change DCX phosphorylation status. Mutation of a consensus phosphorylation site for Dyrk kinases at Serine 306 (Ser306) in DCX indicated that this is one target site for Dyrk2 and Dyrk3. Overexpression of Dyrk2 restored altered DCX distribution in the growth cones of dendrites and axons, and partially reversed the morphological effects of DCX overexpression; some of these effects were abrogated by mutation of Ser306 to alanine. These studies implicate Dyrks in the regulation of cytoskeletal organization and process outgrowth in neurons, and suggest that DCX is one relevant Dyrk target.
Copyright © 2012 Wiley Periodicals, Inc.
Figures





Similar articles
-
Protein quality control of DYRK family protein kinases by the Hsp90-Cdc37 molecular chaperone.Biochim Biophys Acta Mol Cell Res. 2021 Sep;1868(10):119081. doi: 10.1016/j.bbamcr.2021.119081. Epub 2021 Jun 18. Biochim Biophys Acta Mol Cell Res. 2021. PMID: 34147560
-
Splice variants of the dual specificity tyrosine phosphorylation-regulated kinase 4 (DYRK4) differ in their subcellular localization and catalytic activity.J Biol Chem. 2011 Feb 18;286(7):5494-505. doi: 10.1074/jbc.M110.157909. Epub 2010 Dec 2. J Biol Chem. 2011. PMID: 21127067 Free PMC article.
-
Sequence characteristics, subcellular localization, and substrate specificity of DYRK-related kinases, a novel family of dual specificity protein kinases.J Biol Chem. 1998 Oct 2;273(40):25893-902. doi: 10.1074/jbc.273.40.25893. J Biol Chem. 1998. PMID: 9748265
-
Roles of dual specificity tyrosine-phosphorylation-regulated kinase 2 in nervous system development and disease.Front Neurosci. 2022 Sep 8;16:994256. doi: 10.3389/fnins.2022.994256. eCollection 2022. Front Neurosci. 2022. PMID: 36161154 Free PMC article. Review.
-
Structural and functional characteristics of Dyrk, a novel subfamily of protein kinases with dual specificity.Prog Nucleic Acid Res Mol Biol. 1999;62:1-17. doi: 10.1016/s0079-6603(08)60503-6. Prog Nucleic Acid Res Mol Biol. 1999. PMID: 9932450 Review.
Cited by
-
Traffic jams and the complex role of α-Synuclein aggregation in Parkinson disease.Small GTPases. 2017 Apr 3;8(2):78-84. doi: 10.1080/21541248.2016.1199191. Epub 2016 Jun 17. Small GTPases. 2017. PMID: 27314512 Free PMC article. Review.
-
Dual Specificity Kinase DYRK3 Promotes Aggressiveness of Glioblastoma by Altering Mitochondrial Morphology and Function.Int J Mol Sci. 2021 Mar 15;22(6):2982. doi: 10.3390/ijms22062982. Int J Mol Sci. 2021. PMID: 33804169 Free PMC article.
-
Updating dual-specificity tyrosine-phosphorylation-regulated kinase 2 (DYRK2): molecular basis, functions and role in diseases.Cell Mol Life Sci. 2020 Dec;77(23):4747-4763. doi: 10.1007/s00018-020-03556-1. Epub 2020 May 27. Cell Mol Life Sci. 2020. PMID: 32462403 Free PMC article. Review.
-
shRNA-Based Screen Identifies Endocytic Recycling Pathway Components That Act as Genetic Modifiers of Alpha-Synuclein Aggregation, Secretion and Toxicity.PLoS Genet. 2016 Apr 28;12(4):e1005995. doi: 10.1371/journal.pgen.1005995. eCollection 2016 Apr. PLoS Genet. 2016. PMID: 27123591 Free PMC article.
-
Sequential phosphorylation of NDEL1 by the DYRK2-GSK3β complex is critical for neuronal morphogenesis.Elife. 2019 Dec 9;8:e50850. doi: 10.7554/eLife.50850. Elife. 2019. PMID: 31815665 Free PMC article.
References
-
- Alvarez M, Estivill X, de la Luna S. DYRK1A accumulates in splicing speckles through a novel targeting signal and induces speckle disassembly. Journal of cell science. 2003;116:3099–3107. - PubMed
-
- Aranda S, Laguna A, de la Luna S. DYRK family of protein kinases: evolutionary relationships, biochemical properties, and functional roles. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology 2010 - PubMed
-
- Becker W, Joost HG. Structural and functional characteristics of Dyrk, a novel subfamily of protein kinases with dual specificity. [Accessed October 29, 2011];Progress in nucleic acid research and molecular biology. 1999 62:1–17. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9932450. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

