Regulation of NAD(P)H oxidases by AMPK in cardiovascular systems
- PMID: 22357101
- PMCID: PMC3341493
- DOI: 10.1016/j.freeradbiomed.2012.01.025
Regulation of NAD(P)H oxidases by AMPK in cardiovascular systems
Abstract
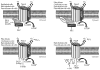
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are ubiquitously produced in cardiovascular systems. Under physiological conditions, ROS/RNS function as signaling molecules that are essential in maintaining cardiovascular function. Aberrant concentrations of ROS/RNS have been demonstrated in cardiovascular diseases owing to increased production or decreased scavenging, which have been considered common pathways for the initiation and progression of cardiovascular diseases such as atherosclerosis, hypertension, (re)stenosis, and congestive heart failure. NAD(P)H oxidases are primary sources of ROS and can be induced or activated by all known cardiovascular risk factors. Stresses, hormones, vasoactive agents, and cytokines via different signaling cascades control the expression and activity of these enzymes and of their regulatory subunits. But the molecular mechanisms by which NAD(P)H oxidase is regulated in cardiovascular systems remain poorly characterized. Investigations by us and others suggest that adenosine monophosphate-activated protein kinase (AMPK), as an energy sensor and modulator, is highly sensitive to ROS/RNS. We have also obtained convincing evidence that AMPK is a physiological suppressor of NAD(P)H oxidase in multiple cardiovascular cell systems. In this review, we summarize our current understanding of how AMPK functions as a physiological repressor of NAD(P)H oxidase.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
AMPKalpha2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo: role of 26S proteasomes.Circ Res. 2010 Apr 2;106(6):1117-28. doi: 10.1161/CIRCRESAHA.109.212530. Epub 2010 Feb 18. Circ Res. 2010. PMID: 20167927 Free PMC article.
-
Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P)H oxidase.Mol Cell Biochem. 2004 Sep;264(1-2):85-97. doi: 10.1023/b:mcbi.0000044378.09409.b5. Mol Cell Biochem. 2004. PMID: 15544038 Review.
-
Neuronal NAD(P)H oxidases contribute to ROS production and mediate RGC death after ischemia.Invest Ophthalmol Vis Sci. 2012 May 14;53(6):2823-30. doi: 10.1167/iovs.12-9526. Invest Ophthalmol Vis Sci. 2012. PMID: 22467573 Free PMC article.
-
NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities.Diabetes Care. 2008 Feb;31 Suppl 2:S170-80. doi: 10.2337/dc08-s247. Diabetes Care. 2008. PMID: 18227481 Review.
-
Resveratrol regulates blood pressure by enhancing AMPK signaling to downregulate a Rac1-derived NADPH oxidase in the central nervous system.J Appl Physiol (1985). 2018 Jul 1;125(1):40-48. doi: 10.1152/japplphysiol.00686.2017. Epub 2018 Mar 1. J Appl Physiol (1985). 2018. PMID: 29494287
Cited by
-
Acetylcholine ameliorates endoplasmic reticulum stress in endothelial cells after hypoxia/reoxygenation via M3 AChR-AMPK signaling.Cell Cycle. 2015 Aug 3;14(15):2461-72. doi: 10.1080/15384101.2015.1060383. Epub 2015 Jun 11. Cell Cycle. 2015. PMID: 26066647 Free PMC article.
-
Association of Oxidative Stress with Neurological Disorders.Curr Neuropharmacol. 2022;20(6):1046-1072. doi: 10.2174/1570159X19666211111141246. Curr Neuropharmacol. 2022. PMID: 34781871 Free PMC article.
-
Adiponectin protects against lung ischemia-reperfusion injury in rats with type 2 diabetes mellitus.Mol Med Rep. 2018 May;17(5):7191-7201. doi: 10.3892/mmr.2018.8748. Epub 2018 Mar 14. Mol Med Rep. 2018. PMID: 29568898 Free PMC article.
-
Adenosine monophosphate-activated protein kinase-α2 deficiency promotes vascular smooth muscle cell migration via S-phase kinase-associated protein 2 upregulation and E-cadherin downregulation.Arterioscler Thromb Vasc Biol. 2013 Dec;33(12):2800-9. doi: 10.1161/ATVBAHA.113.301869. Epub 2013 Oct 10. Arterioscler Thromb Vasc Biol. 2013. Retraction in: Arterioscler Thromb Vasc Biol. 2022 Nov;42(11):e290. doi: 10.1161/ATV.0000000000000158 PMID: 24115035 Free PMC article. Retracted.
-
Whether AICAR in Pregnancy or Lactation Prevents Hypertension Programmed by High Saturated Fat Diet: A Pilot Study.Nutrients. 2020 Feb 11;12(2):448. doi: 10.3390/nu12020448. Nutrients. 2020. PMID: 32053935 Free PMC article.
References
-
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. - PubMed
-
- Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. - PubMed
-
- Wosniak J, Jr, Santos CX, Kowaltowski AJ, Laurindo FR. Cross-talk between mitochondria and NADPH oxidase: effects of mild mitochondrial dysfunction on angiotensin II-mediated increase in Nox isoform expression and activity in vascular smooth muscle cells. Antioxid Redox Signal. 2009;11:1265–1278. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL089920-05/HL/NHLBI NIH HHS/United States
- R01 HL074399/HL/NHLBI NIH HHS/United States
- R01 HL079584-07/HL/NHLBI NIH HHS/United States
- R01 HL079584-09/HL/NHLBI NIH HHS/United States
- R01 HL096032-02/HL/NHLBI NIH HHS/United States
- R01 HL074399-07/HL/NHLBI NIH HHS/United States
- R01 HL079584/HL/NHLBI NIH HHS/United States
- R01 HL074399-08/HL/NHLBI NIH HHS/United States
- R01 HL096032-03/HL/NHLBI NIH HHS/United States
- R01 HL080499-06A1/HL/NHLBI NIH HHS/United States
- R01 HL080499-07/HL/NHLBI NIH HHS/United States
- R01 HL079584-06/HL/NHLBI NIH HHS/United States
- R01 HL110488-01/HL/NHLBI NIH HHS/United States
- R01 HL105157-02/HL/NHLBI NIH HHS/United States
- R01 HL089920/HL/NHLBI NIH HHS/United States
- R01 HL110488/HL/NHLBI NIH HHS/United States
- R01 HL080499-05/HL/NHLBI NIH HHS/United States
- R01 HL105157/HL/NHLBI NIH HHS/United States
- R01 HL105157-03/HL/NHLBI NIH HHS/United States
- R01 HL079584-08/HL/NHLBI NIH HHS/United States
- R01 HL096032-04/HL/NHLBI NIH HHS/United States
- R01 HL080499/HL/NHLBI NIH HHS/United States
- R01 HL105157-04/HL/NHLBI NIH HHS/United States
- R01 HL089920-03/HL/NHLBI NIH HHS/United States
- R01 HL074399-09/HL/NHLBI NIH HHS/United States
- R01 HL110488-02/HL/NHLBI NIH HHS/United States
- R01 HL096032/HL/NHLBI NIH HHS/United States
- R01 HL089920-04/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources

