Maternal LAMP/p55gagHIV-1 DNA immunization induces in utero priming and a long-lasting immune response in vaccinated neonates
- PMID: 22355381
- PMCID: PMC3280311
- DOI: 10.1371/journal.pone.0031608
Maternal LAMP/p55gagHIV-1 DNA immunization induces in utero priming and a long-lasting immune response in vaccinated neonates
Abstract
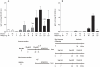
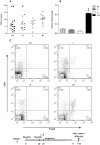
Infants born to HIV-infected mothers are at high risk of becoming infected during gestation or the breastfeeding period. A search is thus warranted for vaccine formulations that will prevent mother-to-child HIV transmission. The LAMP/gag DNA chimeric vaccine encodes the HIV-1 p55gag fused to the lysosome-associated membrane protein-1 (LAMP-1) and has been shown to enhance anti-Gag antibody (Ab) and cellular immune responses in adult and neonatal mice; such a vaccine represents a new concept in antigen presentation. In this study, we evaluated the effect of LAMP/gag DNA immunization on neonates either before conception or during pregnancy. LAMP/gag immunization of BALB/c mice before conception by the intradermal route led to the transfer of anti-Gag IgG1 Ab through the placenta and via breastfeeding. Furthermore, there were an increased percentage of CD4+CD25+Foxp3+T cells in the spleens of neonates. When offspring were immunized with LAMP/gag DNA, the anti-Gag Ab response and the Gag-specific IFN-γ-secreting cells were decreased. Inhibition of anti-Gag Ab production and cellular responses were not observed six months after immunization, indicating that maternal immunization did not interfere with the long-lasting memory response in offspring. Injection of purified IgG in conjunction with LAMP/gag DNA immunization decreased humoral and cytotoxic T-cell responses. LAMP/gag DNA immunization by intradermal injection prior to conception promoted the transfer of Ab, leading to a diminished response to Gag without interfering with the development of anti-Gag T- and B-cell memory. Finally, we assessed responses after one intravenous injection of LAMP/gag DNA during the last five days of pregnancy. The intravenous injection led to in utero immunization. In conclusion, DNA vaccine enconding LAMP-1 with Gag and other HIV-1 antigens should be considered in the development of a protective vaccine for the maternal/fetal and newborn periods.
Conflict of interest statement
Figures
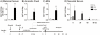
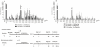


Similar articles
-
Immunization of neonatal mice with LAMP/p55 HIV gag DNA elicits robust immune responses that last to adulthood.Virology. 2010 Oct 10;406(1):37-47. doi: 10.1016/j.virol.2010.06.050. Epub 2010 Jul 29. Virology. 2010. PMID: 20667577
-
Mucosal and systemic anti-GAG immunity induced by neonatal immunization with HIV LAMP/gag DNA vaccine in mice.Immunobiology. 2011 Apr;216(4):505-12. doi: 10.1016/j.imbio.2010.08.007. Epub 2010 Sep 25. Immunobiology. 2011. PMID: 20870310
-
DNA encoding an HIV-1 Gag/human lysosome-associated membrane protein-1 chimera elicits a broad cellular and humoral immune response in Rhesus macaques.PLoS One. 2006 Dec 27;1(1):e135. doi: 10.1371/journal.pone.0000135. PLoS One. 2006. PMID: 17205139 Free PMC article.
-
The protective role of maternal genetic immunization on maternal-fetal health and welfare.Int J Gynaecol Obstet. 2023 Dec;163(3):763-777. doi: 10.1002/ijgo.14853. Epub 2023 May 23. Int J Gynaecol Obstet. 2023. PMID: 37218379 Review.
-
Maternal immune factors involved in the prevention or facilitation of neonatal bacterial infections.Cell Immunol. 2024 Jan-Feb;395-396:104796. doi: 10.1016/j.cellimm.2023.104796. Epub 2023 Dec 7. Cell Immunol. 2024. PMID: 38104514 Review.
Cited by
-
DNA Vaccines Encoding HTNV GP-Derived Th Epitopes Benefited from a LAMP-Targeting Strategy and Established Cellular Immunoprotection.Vaccines (Basel). 2024 Aug 19;12(8):928. doi: 10.3390/vaccines12080928. Vaccines (Basel). 2024. PMID: 39204051 Free PMC article.
-
Enhanced immunogenicity and protective efficacy in mice following a Zika DNA vaccine designed by modulation of membrane-anchoring regions and its association to adjuvants.Front Immunol. 2024 Jan 19;15:1307546. doi: 10.3389/fimmu.2024.1307546. eCollection 2024. Front Immunol. 2024. PMID: 38361945 Free PMC article.
-
LAMP-1 Chimeric to HIV-1 p55Gag in the Immunization of Neonate Mice Induces an Early Germinal Center Formation and AID Expression.Vaccines (Basel). 2022 Aug 3;10(8):1246. doi: 10.3390/vaccines10081246. Vaccines (Basel). 2022. PMID: 36016134 Free PMC article.
-
IgG from Adult Atopic Dermatitis (AD) Patients Induces Nonatopic Neonatal Thymic Gamma-Delta T Cells (γδT) to Acquire IL-22/IL-17 Secretion Profile with Skin-Homing Properties and Epigenetic Implications Mediated by miRNA.Int J Mol Sci. 2022 Jun 20;23(12):6872. doi: 10.3390/ijms23126872. Int J Mol Sci. 2022. PMID: 35743310 Free PMC article.
-
Low doses of IgG from atopic individuals can modulate in vitro IFN-γ production by human intra-thymic TCD4 and TCD8 cells: An IVIg comparative approach.Hum Vaccin Immunother. 2017 Jul 3;13(7):1563-1572. doi: 10.1080/21645515.2017.1299299. Epub 2017 Apr 25. Hum Vaccin Immunother. 2017. PMID: 28441069 Free PMC article.
References
-
- UNAIDS. AIDS Epidemic Update. 2010. Available: http://www.unaids.org/globalreport/Global_report.htm. Accessed 2011 Jan 15.
-
- John GC, Nduati RW, Mbori-Ngacha DA, Richardson BA, Panteleeff D, et al. Correlates of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission: association with maternal plasma HIV-1 RNA load, genital HIV-1 DNA shedding, and breast infections. J Infect Dis. 2001;183:206–212. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

