Detection and characterisation of multi-drug resistance protein 1 (MRP-1) in human mitochondria
- PMID: 22353810
- PMCID: PMC3304412
- DOI: 10.1038/bjc.2012.40
Detection and characterisation of multi-drug resistance protein 1 (MRP-1) in human mitochondria
Abstract
Background: Overexpression of plasma membrane multi-drug resistance protein 1 (MRP-1) can lead to multidrug resistance. In this study, we describe for the first time the expression of mitochondrial MRP-1 in untreated human normal and cancer cells and tissues.
Methods: MRP-1 expression and subcellular localisation in normal and cancer cells and tissues was examined by differential centrifugation and western blotting, and immunofluorescence microscopy. Viable mitochondria were isolated and MRP-1 efflux activity measured using the calcein-AM functional assay. MRP-1 expression was increased using retroviral infection and specific overexpression confirmed by RNA array. Cell viability was determined by trypan blue exclusion and annexin V-propidium iodide labelling of cells.
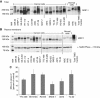
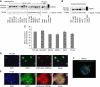
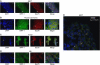
Results: MRP-1 was detected in the mitochondria of cancer and normal cells and tissues. The efflux activity of mitochondrial MRP-1 was more efficient (55-64%) than that of plasma membrane MRP-1 (11-22%; P<0.001). Induced MRP-1 expression resulted in a preferential increase in mitochondrial MRP-1, suggesting selective targeting to this organelle. Treatment with a non-lethal concentration of doxorubicin (0.85 nM, 8 h) increased mitochondrial and plasma membrane MRP-1, increasing resistance to MRP-1 substrates. For the first time, we have identified MRP-1 with efflux activity in human mitochondria.
Conclusion: Mitochondrial MRP-1 may be an exciting new therapeutic target where historically MRP-1 inhibitor strategies have limited clinical success.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Localization of MRP-1 to the outer mitochondrial membrane by the chaperone protein HSP90β.FASEB J. 2016 May;30(5):1712-23. doi: 10.1096/fj.15-283408. Epub 2015 Dec 31. FASEB J. 2016. PMID: 26722004
-
Kinetic analysis of calcein and calcein-acetoxymethylester efflux mediated by the multidrug resistance protein and P-glycoprotein.Biochemistry. 1998 Feb 24;37(8):2243-50. doi: 10.1021/bi9718043. Biochemistry. 1998. PMID: 9485370
-
Multidrug resistance protein (MRP)-mediated transport of leukotriene C4 and chemotherapeutic agents in membrane vesicles. Demonstration of glutathione-dependent vincristine transport.J Biol Chem. 1996 Apr 19;271(16):9675-82. doi: 10.1074/jbc.271.16.9675. J Biol Chem. 1996. PMID: 8621643
-
Interactions of ofloxacin and erythromycin with the multidrug resistance protein (MRP) in MRP-overexpressing human leukemia cells.Antimicrob Agents Chemother. 2000 Jun;44(6):1697-700. doi: 10.1128/AAC.44.6.1697-1700.2000. Antimicrob Agents Chemother. 2000. PMID: 10817732 Free PMC article.
-
Multidrug resistance-associated protein: a protein distinct from P-glycoprotein involved in cytotoxic drug expulsion.Gen Pharmacol. 1997 May;28(5):639-45. doi: 10.1016/s0306-3623(96)00284-4. Gen Pharmacol. 1997. PMID: 9184795 Review.
Cited by
-
Membrane expression of MRP-1, but not MRP-1 splicing or Pgp expression, predicts survival in patients with ESFT.Br J Cancer. 2013 Jul 9;109(1):195-206. doi: 10.1038/bjc.2013.168. Epub 2013 Jun 25. Br J Cancer. 2013. PMID: 23799853 Free PMC article.
-
Germacrone reverses Adriamycin resistance through cell apoptosis in multidrug-resistant breast cancer cells.Exp Ther Med. 2014 Nov;8(5):1611-1615. doi: 10.3892/etm.2014.1932. Epub 2014 Aug 26. Exp Ther Med. 2014. PMID: 25289068 Free PMC article.
-
High-content screening of drug combinations of an mPGES-1 inhibitor in multicellular tumor spheroids leads to mechanistic insights into neuroblastoma chemoresistance.Mol Oncol. 2024 Feb;18(2):317-335. doi: 10.1002/1878-0261.13502. Epub 2023 Aug 21. Mol Oncol. 2024. PMID: 37519014 Free PMC article.
-
Mitochondria of a human multidrug-resistant hepatocellular carcinoma cell line constitutively express inducible nitric oxide synthase in the inner membrane.J Cell Mol Med. 2015 Jun;19(6):1410-7. doi: 10.1111/jcmm.12528. Epub 2015 Feb 18. J Cell Mol Med. 2015. PMID: 25691007 Free PMC article.
-
Linker Domains: Why ABC Transporters 'Live in Fragments no Longer'.Trends Biochem Sci. 2020 Feb;45(2):137-148. doi: 10.1016/j.tibs.2019.11.004. Epub 2019 Dec 12. Trends Biochem Sci. 2020. PMID: 31839525 Free PMC article. Review.
References
-
- Chapman EJ, Hurst CD, Pitt E, Chambers P, Aveyard JS, Knowles MA (2006) Expression of hTERT immortalises normal human urothelial cells without inactivation of the p16/Rb pathway. Oncogene 25: 5037–5045 - PubMed
-
- Cheon M, Park D, Park Y, Kam K, Park SD, Ryu K (2000) Homologous upregulation of gonadotropin-releasing hormone receptor mRNA occurs through transcriptional activation rather than modulation of mRNA stability. Endocrine 13: 47–53 - PubMed
-
- Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM, Deeley RG (1992) Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 258: 1650–1654 - PubMed
-
- Gandhi L, Harding MW, Neubauer M, Langer CJ, Moore M, Ross HJ, Johnson BE, Lynch TJ (2007) A phase II study of the safety and efficacy of the multidrug resistance inhibitor VX-710 combined with doxorubicin and vincristine in patients with recurrent small cell lung cancer. Cancer 109: 924–932 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

