Arf6 promotes autophagosome formation via effects on phosphatidylinositol 4,5-bisphosphate and phospholipase D
- PMID: 22351926
- PMCID: PMC3283994
- DOI: 10.1083/jcb.201110114
Arf6 promotes autophagosome formation via effects on phosphatidylinositol 4,5-bisphosphate and phospholipase D
Abstract
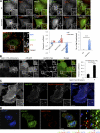
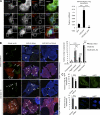
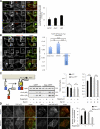
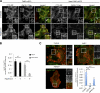
Macroautophagy (in this paper referred to as autophagy) and the ubiquitin-proteasome system are the two major catabolic systems in cells. Autophagy involves sequestration of cytosolic contents in double membrane-bounded vesicles called autophagosomes. The membrane source for autophagosomes has received much attention, and diverse sources, such as the plasma membrane, Golgi, endoplasmic reticulum, and mitochondria, have been implicated. These may not be mutually exclusive, but the exact sources and mechanism involved in the formation of autophagosomes are still unclear. In this paper, we identify a positive role for the small G protein Arf6 in autophagosome formation. The effect of Arf6 on autophagy is mediated by its role in the generation of phosphatidylinositol 4,5-bisphosphate (PIP(2)) and in inducing phospholipase D (PLD) activity. PIP(2) and PLD may themselves promote autophagosome biogenesis by influencing endocytic uptake of plasma membrane into autophagosome precursors. However, Arf6 may also influence autophagy by indirect effects, such as either by regulating membrane flow from other compartments or by modulating PLD activity independently of the mammalian target of rapamycin.
Figures

Similar articles
-
Localization and regulation of phospholipase D2 by ARF6.J Cell Biochem. 2005 May 1;95(1):149-64. doi: 10.1002/jcb.20351. J Cell Biochem. 2005. PMID: 15759270
-
Mechanism of ADP ribosylation factor-stimulated phosphatidylinositol 4,5-bisphosphate synthesis in HL60 cells.J Biol Chem. 2002 Feb 22;277(8):5823-31. doi: 10.1074/jbc.M110274200. Epub 2001 Dec 14. J Biol Chem. 2002. PMID: 11744730
-
ADP-ribosylation factor 6 regulates mammalian myoblast fusion through phospholipase D1 and phosphatidylinositol 4,5-bisphosphate signaling pathways.Mol Biol Cell. 2010 Jul 15;21(14):2412-24. doi: 10.1091/mbc.e09-12-1063. Epub 2010 May 26. Mol Biol Cell. 2010. PMID: 20505075 Free PMC article.
-
The autophagosome: origins unknown, biogenesis complex.Nat Rev Mol Cell Biol. 2013 Dec;14(12):759-74. doi: 10.1038/nrm3696. Epub 2013 Nov 8. Nat Rev Mol Cell Biol. 2013. PMID: 24201109 Review.
-
Mechanisms of autophagosome biogenesis.Curr Biol. 2012 Jan 10;22(1):R29-34. doi: 10.1016/j.cub.2011.11.034. Curr Biol. 2012. PMID: 22240478 Review.
Cited by
-
Arf6 controls beta-amyloid production by regulating macropinocytosis of the Amyloid Precursor Protein to lysosomes.Mol Brain. 2015 Jul 14;8:41. doi: 10.1186/s13041-015-0129-7. Mol Brain. 2015. PMID: 26170135 Free PMC article.
-
Evidence for Involvement of ADP-Ribosylation Factor 6 in Intracellular Trafficking and Release of Murine Leukemia Virus Gag.Cells. 2024 Jan 31;13(3):270. doi: 10.3390/cells13030270. Cells. 2024. PMID: 38334661 Free PMC article.
-
Host Cell Signatures of the Envelopment Site within Beta-Herpes Virions.Int J Mol Sci. 2022 Sep 1;23(17):9994. doi: 10.3390/ijms23179994. Int J Mol Sci. 2022. PMID: 36077391 Free PMC article. Review.
-
TDP-43 loss of function inhibits endosomal trafficking and alters trophic signaling in neurons.EMBO J. 2016 Nov 2;35(21):2350-2370. doi: 10.15252/embj.201694221. Epub 2016 Sep 12. EMBO J. 2016. PMID: 27621269 Free PMC article.
-
Binding of PLD2-Generated Phosphatidic Acid to KIF5B Promotes MT1-MMP Surface Trafficking and Lung Metastasis of Mouse Breast Cancer Cells.Dev Cell. 2017 Oct 23;43(2):186-197.e7. doi: 10.1016/j.devcel.2017.09.012. Epub 2017 Oct 12. Dev Cell. 2017. PMID: 29033361 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

