Impaired autoproteolytic cleavage of mCLCA6, a murine integral membrane protein expressed in enterocytes, leads to cleavage at the plasma membrane instead of the endoplasmic reticulum
- PMID: 22350745
- PMCID: PMC3887709
- DOI: 10.1007/s10059-012-2217-1
Impaired autoproteolytic cleavage of mCLCA6, a murine integral membrane protein expressed in enterocytes, leads to cleavage at the plasma membrane instead of the endoplasmic reticulum
Abstract
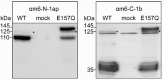
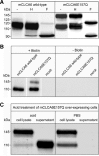
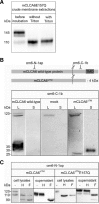
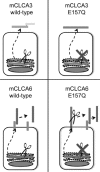
CLCA proteins (calcium-activated chloride channel regulators) have been linked to diseases involving secretory disorders, including cystic fibrosis (CF) and asthma. They have been shown to modulate endogenous chloride conductance, possibly by acting as metalloproteases. Based on the differential processing of the subunits after posttranslational cleavage, two subgroups of CLCA proteins can be distinguished. In one subgroup, both subunits are secreted, in the other group, the carboxy-terminal subunit possesses a transmembrane segment, resulting in shedding of only the amino-terminal subunit. Recent data on the post-translational cleavage and proteolytic activity of CLCA are limited to secreted CLCA. In this study, we characterized the cleavage of mCLCA6, a murine CLCA possessing a transmembrane segment. As for secreted CLCA, the cleavage in the endoplasmic reticulum was not observed for a protein with the E157Q mutation in the HEXXH motif of mCLCA6, suggesting that this mutant protein and secreted CLCA family members share a similar autoproteolytic cleavage mechanism. In contrast to secreted CLCA proteins with the E157Q mutation, the uncleaved precursor of the mCLCA6E157Q mutant reached the plasma membrane, where it was cleaved and the amino-terminal subunit was shed into the supernatant. Using crude membrane fractions, we showed that cleavage of the mCLCA6E157Q protein is zinc-dependent and sensitive to metalloprotease inhibitors, suggesting secondary cleavage by a metalloprotease. Interestingly, anchorage of mCLCA6E157Q to the plasma membrane is not essential for its secondary cleavage, because the mCLCA6(Δ™)E157Q mutant still underwent cleavage. Our data suggest that the processing of CLCA proteins is more complex than previously recognized.
Figures

Similar articles
-
The murine goblet cell protein mCLCA3 is a zinc-dependent metalloprotease with autoproteolytic activity.Mol Cells. 2011 Dec;32(6):535-41. doi: 10.1007/s10059-011-0158-8. Epub 2011 Nov 9. Mol Cells. 2011. PMID: 22080371 Free PMC article.
-
Murine mCLCA6 is an integral apical membrane protein of non-goblet cell enterocytes and co-localizes with the cystic fibrosis transmembrane conductance regulator.J Histochem Cytochem. 2008 May;56(5):495-509. doi: 10.1369/jhc.2008.950592. Epub 2008 Feb 18. J Histochem Cytochem. 2008. PMID: 18285349 Free PMC article.
-
Self-cleavage of human CLCA1 protein by a novel internal metalloprotease domain controls calcium-activated chloride channel activation.J Biol Chem. 2012 Dec 7;287(50):42138-49. doi: 10.1074/jbc.M112.410282. Epub 2012 Oct 30. J Biol Chem. 2012. PMID: 23112050 Free PMC article.
-
The CLCA gene family: putative therapeutic target for respiratory diseases.Inflamm Allergy Drug Targets. 2009 Jun;8(2):146-60. doi: 10.2174/187152809788462590. Inflamm Allergy Drug Targets. 2009. PMID: 19530997 Review.
-
Structure and function of CLCA proteins.Physiol Rev. 2005 Jul;85(3):1061-92. doi: 10.1152/physrev.00016.2004. Physiol Rev. 2005. PMID: 15987802 Review.
Cited by
-
Evolutionarily conserved properties of CLCA proteins 1, 3 and 4, as revealed by phylogenetic and biochemical studies in avian homologues.PLoS One. 2022 Apr 13;17(4):e0266937. doi: 10.1371/journal.pone.0266937. eCollection 2022. PLoS One. 2022. PMID: 35417490 Free PMC article.
-
Self-Cleavage of Human Chloride Channel Accessory 2 Causes a Conformational Shift That Depends on Membrane Anchorage and Is Required for Its Regulation of Store-Operated Calcium Entry.Biomedicines. 2023 Oct 28;11(11):2915. doi: 10.3390/biomedicines11112915. Biomedicines. 2023. PMID: 38001916 Free PMC article.
-
IL-13-induced airway mucus production is attenuated by MAPK13 inhibition.J Clin Invest. 2012 Dec;122(12):4555-68. doi: 10.1172/JCI64896. Epub 2012 Nov 26. J Clin Invest. 2012. PMID: 23187130 Free PMC article.
-
CLCAs - a family of metalloproteases of intriguing phylogenetic distribution and with cases of substituted catalytic sites.PLoS One. 2013 May 9;8(5):e62272. doi: 10.1371/journal.pone.0062272. Print 2013. PLoS One. 2013. PMID: 23671590 Free PMC article.
-
SheddomeDB: the ectodomain shedding database for membrane-bound shed markers.BMC Bioinformatics. 2017 Mar 14;18(Suppl 3):42. doi: 10.1186/s12859-017-1465-7. BMC Bioinformatics. 2017. PMID: 28361715 Free PMC article. Review.
References
-
- Braun J., Bothe M.K., Mundhenk L., Beck C.L., Gruber A.D. Murine mCLCA5 is expressed in granular layer keratinocytes of stratified epithelia. Histochem. Cell Biol. 2010;133:285–299. - PubMed
-
- Cha J., Auld D.S. Site-directed mutagenesis of the active site glutamate in human matrilysin: investigation of its role in catalysis. Biochemistry. 1997;36:16019–16024. - PubMed
-
- Elble R.C., Walia V., Cheng H.C., Connon C.J., Mundhenk L., Gruber A.D., Pauli B. U. The putative chloride channel hCLCA2 has a single C-terminal transmembrane segment. J. Biol. Chem. 2006;281:29448–29454. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

