In vivo imaging of immunotoxin treatment using Katushka-transfected A-431 cells in a murine xenograft tumour model
- PMID: 22350071
- PMCID: PMC11028735
- DOI: 10.1007/s00262-012-1219-3
In vivo imaging of immunotoxin treatment using Katushka-transfected A-431 cells in a murine xenograft tumour model
Abstract
Purpose: Preclinical in vivo analyses of treatment responses are an important prerequisite to evaluate new therapeutics. Molecular in vivo imaging in the far red (FR)/near infra red (NIR) is a promising method, as it enables measurements at different time points in individual animals, thereby reducing the number of animals required, while increasing statistical significance. Here, we show the establishment of a method to monitor response to treatment using fluorescent cells, expressing the epidermal growth factor receptor (EGFR), a target already used in therapy.
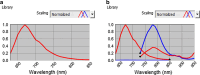
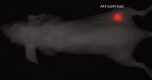
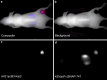
Methods: We transfected A-431 tumour cells with the far red-emitting protein Katushka (Kat2), resulting in strong fluorescence allowing for the monitoring of tumour growth when implanted in BALB/c nu/nu mice with a CRi Maestro in vivo imager. We targeted A-431 cells with a previously reported immunotoxin (IT), consisting of the anti-EGFR antibody single-chain variable fragment (scFv) 425, fused to Pseudomonas aeruginosa Exotoxin A' (ETA'). In addition, EGFR expression was verified using the 425(scFv) conjugated to a NIR dye BG-747 through a SNAP-tag linker.
Results: The results show the feasibility to evaluate response to treatment in vivo by FR imaging, while at the same location detecting EGFR expression. Treatment with 425(scFv)-ETA' resulted in decelerated tumour growth, while not affecting the overall health of the animals. This is in contrast to treatment with Doxorubicin, which, although decreasing the tumour size, resulted in poor health.
Conclusions: We developed a novel method to non-invasively determine treatment responses by in vivo imaging of multiple parameters which showed the efficacy of 425(scFv)-ETA'.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Recombinant anti-EGFR immunotoxin 425(scFv)-ETA' demonstrates anti-tumor activity against disseminated human pancreatic cancer in nude mice.Int J Mol Med. 2005 Feb;15(2):305-13. Int J Mol Med. 2005. PMID: 15647848
-
Targeting tumor-associated macrophages in an experimental glioma model with a recombinant immunotoxin to folate receptor beta.Cancer Immunol Immunother. 2009 Oct;58(10):1577-86. doi: 10.1007/s00262-009-0667-x. Epub 2009 Feb 24. Cancer Immunol Immunother. 2009. PMID: 19238383 Free PMC article.
-
Human kappa light chain targeted Pseudomonas exotoxin A--identifying human antibodies and Fab fragments with favorable characteristics for antibody-drug conjugate development.J Immunol Methods. 2011 Aug 31;371(1-2):122-33. doi: 10.1016/j.jim.2011.06.023. Epub 2011 Jun 30. J Immunol Methods. 2011. PMID: 21756911
-
Immunotoxins in the treatment of refractory hairy cell leukemia.Hematol Oncol Clin North Am. 2006 Oct;20(5):1137-51, viii. doi: 10.1016/j.hoc.2006.06.009. Hematol Oncol Clin North Am. 2006. PMID: 16990113 Review.
-
Cintredekin besudotox in treatment of malignant glioma.Expert Opin Biol Ther. 2008 Jun;8(6):805-12. doi: 10.1517/14712598.8.6.805. Expert Opin Biol Ther. 2008. PMID: 18476792 Review.
Cited by
-
HER2-Specific Targeted Toxin DARPin-LoPE: Immunogenicity and Antitumor Effect on Intraperitoneal Ovarian Cancer Xenograft Model.Int J Mol Sci. 2019 May 15;20(10):2399. doi: 10.3390/ijms20102399. Int J Mol Sci. 2019. PMID: 31096563 Free PMC article.
-
Novel EGFR-specific immunotoxins based on panitumumab and cetuximab show in vitro and ex vivo activity against different tumor entities.J Cancer Res Clin Oncol. 2015 Dec;141(12):2079-95. doi: 10.1007/s00432-015-1975-5. Epub 2015 Apr 22. J Cancer Res Clin Oncol. 2015. PMID: 25899161
-
Targeted Delivery to Tumors: Multidirectional Strategies to Improve Treatment Efficiency.Cancers (Basel). 2019 Jan 10;11(1):68. doi: 10.3390/cancers11010068. Cancers (Basel). 2019. PMID: 30634580 Free PMC article. Review.
-
In vitro effects and ex vivo binding of an EGFR-specific immunotoxin on rhabdomyosarcoma cells.J Cancer Res Clin Oncol. 2015 Jun;141(6):1049-61. doi: 10.1007/s00432-014-1884-z. Epub 2014 Nov 30. J Cancer Res Clin Oncol. 2015. PMID: 25433506
-
Applications of SNAP-tag technology in skin cancer therapy.Health Sci Rep. 2019 Jan 8;2(2):e103. doi: 10.1002/hsr2.103. eCollection 2019 Feb. Health Sci Rep. 2019. PMID: 30809593 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

