Basolateral sorting of the coxsackie and adenovirus receptor through interaction of a canonical YXXPhi motif with the clathrin adaptors AP-1A and AP-1B
- PMID: 22343291
- PMCID: PMC3309744
- DOI: 10.1073/pnas.1117949109
Basolateral sorting of the coxsackie and adenovirus receptor through interaction of a canonical YXXPhi motif with the clathrin adaptors AP-1A and AP-1B
Abstract
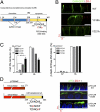
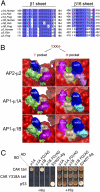
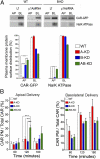
The coxsackie and adenovirus receptor (CAR) plays key roles in epithelial barrier function at the tight junction, a localization guided in part by a tyrosine-based basolateral sorting signal, (318)YNQV(321). Sorting motifs of this type are known to route surface receptors into clathrin-mediated endocytosis through interaction with the medium subunit (μ2) of the clathrin adaptor AP-2, but how they guide new and recycling membrane proteins basolaterally is unknown. Here, we show that YNQV functions as a canonical YxxΦ motif, with both Y318 and V321 required for the correct basolateral localization and biosynthetic sorting of CAR, and for interaction with a highly conserved pocket in the medium subunits (μ1A and μ1B) of the clathrin adaptors AP-1A and AP-1B. Knock-down experiments demonstrate that AP-1A plays a role in the biosynthetic sorting of CAR, complementary to the role of AP-1B in basolateral recycling of this receptor. Our study illustrates how two clathrin adaptors direct basolateral trafficking of a plasma membrane protein through interaction with a canonical YxxΦ motif.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The clathrin adaptor AP-1A mediates basolateral polarity.Dev Cell. 2012 Apr 17;22(4):811-23. doi: 10.1016/j.devcel.2012.02.004. Dev Cell. 2012. PMID: 22516199 Free PMC article.
-
Basolateral sorting of the Mg²⁺ transporter CNNM4 requires interaction with AP-1A and AP-1B.Biochem Biophys Res Commun. 2014 Dec 12;455(3-4):184-9. doi: 10.1016/j.bbrc.2014.10.138. Epub 2014 Nov 10. Biochem Biophys Res Commun. 2014. PMID: 25449265
-
The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains.J Cell Biol. 2003 Oct 27;163(2):351-62. doi: 10.1083/jcb.200309020. J Cell Biol. 2003. PMID: 14581457 Free PMC article.
-
Clathrin and AP1B: key roles in basolateral trafficking through trans-endosomal routes.FEBS Lett. 2009 Dec 3;583(23):3784-95. doi: 10.1016/j.febslet.2009.10.050. Epub 2009 Oct 23. FEBS Lett. 2009. PMID: 19854182 Free PMC article. Review.
-
Role of the epithelial cell-specific clathrin adaptor complex AP-1B in cell polarity.Cell Logist. 2015 Jul 30;5(2):e1074331. doi: 10.1080/21592799.2015.1074331. eCollection 2015 Apr-Jun. Cell Logist. 2015. PMID: 27057418 Free PMC article. Review.
Cited by
-
Disruption of the coxsackievirus and adenovirus receptor-homodimeric interaction triggers lipid microdomain- and dynamin-dependent endocytosis and lysosomal targeting.J Biol Chem. 2014 Jan 10;289(2):680-95. doi: 10.1074/jbc.M113.518365. Epub 2013 Nov 22. J Biol Chem. 2014. PMID: 24273169 Free PMC article.
-
Adenovirus transduction: More complicated than receptor expression.Virology. 2017 Feb;502:144-151. doi: 10.1016/j.virol.2016.12.020. Epub 2016 Dec 31. Virology. 2017. PMID: 28049062 Free PMC article.
-
A sorting nexin 17-binding domain within the LRP1 cytoplasmic tail mediates receptor recycling through the basolateral sorting endosome.Traffic. 2013 Jul;14(7):823-38. doi: 10.1111/tra.12076. Epub 2013 May 8. Traffic. 2013. PMID: 23593972 Free PMC article.
-
Coxsackievirus and adenovirus receptor (CAR) mediates trafficking of acid sensing ion channel 3 (ASIC3) via PSD-95.Biochem Biophys Res Commun. 2012 Aug 17;425(1):13-8. doi: 10.1016/j.bbrc.2012.07.033. Epub 2012 Jul 15. Biochem Biophys Res Commun. 2012. PMID: 22809504 Free PMC article.
-
ADAPTOR PROTEIN-1 complex-mediated post-Golgi trafficking is critical for pollen wall development in Arabidopsis.New Phytol. 2022 Jul;235(2):472-487. doi: 10.1111/nph.18170. Epub 2022 May 21. New Phytol. 2022. PMID: 35451504 Free PMC article.
References
-
- Freimuth P, Philipson L, Carson SD. The coxsackievirus and adenovirus receptor. Curr Top Microbiol Immunol. 2008;323:67–87. - PubMed
-
- Bergelson JM, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. - PubMed
-
- Cohen CJ, Gaetz J, Ohman T, Bergelson JM. Multiple regions within the coxsackievirus and adenovirus receptor cytoplasmic domain are required for basolateral sorting. J Biol Chem. 2001;276:25392–25398. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

