Pumilio 1 suppresses multiple activators of p53 to safeguard spermatogenesis
- PMID: 22342750
- PMCID: PMC3449084
- DOI: 10.1016/j.cub.2012.01.039
Pumilio 1 suppresses multiple activators of p53 to safeguard spermatogenesis
Abstract
During spermatogenesis, germ cells initially expand exponentially through mitoses. A majority of these cells are then eliminated through p53-mediated apoptosis to maintain germline homeostasis. However, the activity of p53 must be precisely modulated, especially suppressed in postmitotic spermatogenic cells, to guarantee robustness of spermatogenesis. Currently, how the suppression is achieved is not understood. Here, we show that Pumilio 1, a posttranscriptional regulator, binds to mRNAs representing 1,527 genes, with significant enrichment for mRNAs involved in pathways regulating p53, cell cycle, and MAPK signaling. In particular, eight mRNAs encoding activators of p53 are repressed by Pumilio 1. Deleting Pumilio 1 results in strong activation of p53 and apoptosis mostly in spermatocytes, which disrupts sperm production and fertility. Removing p53 reduces apoptosis and rescues testicular hypotrophy in Pumilio 1 null mice. These results indicate that key components of the p53 pathway are coordinately regulated by Pumilio 1 at the posttranscriptional level, which may exemplify an RNA operon.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
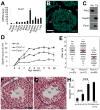
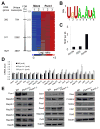
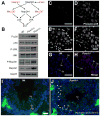
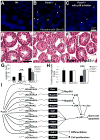
Comment in
-
Pumilio 1 control of spermatogenesis: a roadmap for future research.Asian J Androl. 2012 Sep;14(5):669. doi: 10.1038/aja.2012.38. Epub 2012 Jun 18. Asian J Androl. 2012. PMID: 22705564 Free PMC article. No abstract available.
Similar articles
-
Interplay between p53 and Ink4c in spermatogenesis and fertility.Cell Cycle. 2018;17(5):643-651. doi: 10.1080/15384101.2017.1421874. Epub 2018 Feb 22. Cell Cycle. 2018. PMID: 29334315 Free PMC article.
-
p53-mediated germ cell quality control in spermatogenesis.Dev Biol. 1998 Dec 1;204(1):165-71. doi: 10.1006/dbio.1998.9074. Dev Biol. 1998. PMID: 9851850
-
YTHDF2 is essential for spermatogenesis and fertility by mediating a wave of transcriptional transition in spermatogenic cells.Acta Biochim Biophys Sin (Shanghai). 2021 Dec 8;53(12):1702-1712. doi: 10.1093/abbs/gmab148. Acta Biochim Biophys Sin (Shanghai). 2021. PMID: 34664060
-
Posttranscriptional regulation of p53 and its targets by RNA-binding proteins.Curr Mol Med. 2008 Dec;8(8):845-9. doi: 10.2174/156652408786733748. Curr Mol Med. 2008. PMID: 19075680 Free PMC article. Review.
-
[RNA-binding protein PTB in spermatogenesis: Progress in studies].Zhonghua Nan Ke Xue. 2016 Sep;22(9):856-860. Zhonghua Nan Ke Xue. 2016. PMID: 29071887 Review. Chinese.
Cited by
-
Human Pumilio proteins recruit multiple deadenylases to efficiently repress messenger RNAs.J Biol Chem. 2012 Oct 19;287(43):36370-83. doi: 10.1074/jbc.M112.373522. Epub 2012 Sep 6. J Biol Chem. 2012. PMID: 22955276 Free PMC article.
-
SPIN1 is a proto-oncogene and SPIN3 is a tumor suppressor in human seminoma.Oncotarget. 2018 Aug 21;9(65):32466-32477. doi: 10.18632/oncotarget.25977. eCollection 2018 Aug 21. Oncotarget. 2018. PMID: 30197756 Free PMC article.
-
Variation analysis of PUM1 gene in Chinese women with primary ovarian insufficiency.J Assist Reprod Genet. 2018 Apr;35(4):727-731. doi: 10.1007/s10815-017-1110-4. Epub 2018 Jan 3. J Assist Reprod Genet. 2018. PMID: 29297114 Free PMC article.
-
Post-transcriptional gene expression control by NANOS is up-regulated and functionally important in pRb-deficient cells.EMBO J. 2014 Oct 1;33(19):2201-15. doi: 10.15252/embj.201488057. Epub 2014 Aug 6. EMBO J. 2014. PMID: 25100735 Free PMC article.
-
Driving apoptosis-relevant proteins toward neural differentiation.Mol Neurobiol. 2012 Oct;46(2):316-31. doi: 10.1007/s12035-012-8289-2. Epub 2012 Jul 1. Mol Neurobiol. 2012. PMID: 22752662 Review.
References
-
- Yin Y, Stahl BC, DeWolf WC, Morgentaler A. P53 and Fas are sequential mechanisms of testicular germ cell apoptosis. J Androl. 2002;23:64–70. - PubMed
-
- Russell LD, Chiarini-Garcia H, Korsmeyer SJ, Knudson CM. Bax-dependent spermatogonia apoptosis is required for testicular development and spermatogenesis. Biol Reprod. 2002;66:950–958. - PubMed
-
- Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270:96–99. - PubMed
-
- Beumer TL, Roepers-Gajadien HL, Gademan IS, van Buul PP, Gil-Gomez G, Rutgers DH, de Rooij DG. The role of the tumor suppressor p53 in spermatogenesis. Cell Death Differ. 1998;5:669–677. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

