Multiple forms of Spire-actin complexes and their functional consequences
- PMID: 22334675
- PMCID: PMC3323035
- DOI: 10.1074/jbc.M111.317792
Multiple forms of Spire-actin complexes and their functional consequences
Abstract
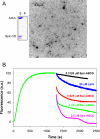
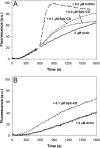
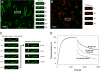
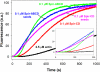
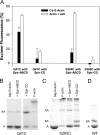
Spire is a WH2 domain-containing actin nucleator essential for establishing an actin mesh during oogenesis. In vitro, in addition to nucleating filaments, Spire can sever them and sequester actin monomers. Understanding how Spire is capable of these disparate functions and which are physiologically relevant is an important goal. To study severing, we examined the effect of Drosophila Spire on preformed filaments in bulk and single filament assays. We observed rapid depolymerization of actin filaments by Spire, which we conclude is largely due to its sequestration activity and enhanced by its weak severing activity. We also studied the solution and crystal structures of Spire-actin complexes. We find structural and functional differences between constructs containing four WH2 domains (Spir-ABCD) and two WH2 domains (Spir-CD) that may provide insight into the mechanisms of nucleation and sequestration. Intriguingly, we observed lateral interactions between actin monomers associated with Spir-ABCD, suggesting that the structures built by these four tandem WH2 domains are more complex than originally imagined. Finally, we propose that Spire-actin mixtures contain both nuclei and sequestration structures.
Figures
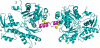
Similar articles
-
Molecular architecture of the Spire-actin nucleus and its implication for actin filament assembly.Proc Natl Acad Sci U S A. 2011 Dec 6;108(49):19575-80. doi: 10.1073/pnas.1115465108. Epub 2011 Nov 21. Proc Natl Acad Sci U S A. 2011. PMID: 22106272 Free PMC article.
-
Drosophila Spire is an actin nucleation factor.Nature. 2005 Jan 27;433(7024):382-8. doi: 10.1038/nature03241. Nature. 2005. PMID: 15674283
-
Filament assembly by Spire: key residues and concerted actin binding.J Mol Biol. 2015 Feb 27;427(4):824-839. doi: 10.1016/j.jmb.2014.09.002. Epub 2014 Sep 16. J Mol Biol. 2015. PMID: 25234086 Free PMC article.
-
Cellular functions of the Spir actin-nucleation factors.Trends Cell Biol. 2006 Sep;16(9):477-83. doi: 10.1016/j.tcb.2006.07.005. Epub 2006 Aug 9. Trends Cell Biol. 2006. PMID: 16901698 Review.
-
Actin nucleation: spire - actin nucleator in a class of its own.Curr Biol. 2005 Apr 26;15(8):R305-8. doi: 10.1016/j.cub.2005.04.004. Curr Biol. 2005. PMID: 15854898 Review.
Cited by
-
Structure of a Bud6/Actin Complex Reveals a Novel WH2-like Actin Monomer Recruitment Motif.Structure. 2015 Aug 4;23(8):1492-1499. doi: 10.1016/j.str.2015.05.015. Epub 2015 Jun 25. Structure. 2015. PMID: 26118535 Free PMC article.
-
Zero-mode waveguides visualize the first steps during gelsolin-mediated actin filament formation.Biophys J. 2022 Jan 18;121(2):327-335. doi: 10.1016/j.bpj.2021.12.011. Epub 2021 Dec 9. Biophys J. 2022. PMID: 34896371 Free PMC article.
-
Direct interaction between two actin nucleators is required in Drosophila oogenesis.Development. 2013 Nov;140(21):4417-25. doi: 10.1242/dev.097337. Epub 2013 Oct 2. Development. 2013. PMID: 24089467 Free PMC article.
-
Actin filament assembly by bacterial factors VopL/F: Which end is up?J Cell Biol. 2017 May 1;216(5):1211-1213. doi: 10.1083/jcb.201702165. Epub 2017 Apr 17. J Cell Biol. 2017. PMID: 28416477 Free PMC article.
-
In vitro studies of actin filament and network dynamics.Curr Opin Cell Biol. 2013 Feb;25(1):6-13. doi: 10.1016/j.ceb.2012.11.007. Epub 2012 Dec 22. Curr Opin Cell Biol. 2013. PMID: 23267766 Free PMC article. Review.
References
-
- Quinlan M. E., Heuser J. E., Kerkhoff E., Mullins R. D. (2005) Drosophila Spire is an actin nucleation factor. Nature 433, 382–388 - PubMed
-
- Tam V. C., Serruto D., Dziejman M., Brieher W., Mekalanos J. J. (2007) A type III secretion system in Vibrio cholerae translocates a formin/spire hybrid-like actin nucleator to promote intestinal colonization. Cell Host Microbe 1, 95–107 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

