Guanine nucleotide-binding protein Gαi2: a new partner of claudin-5 that regulates tight junction integrity in human brain endothelial cells
- PMID: 22333621
- PMCID: PMC3345908
- DOI: 10.1038/jcbfm.2011.202
Guanine nucleotide-binding protein Gαi2: a new partner of claudin-5 that regulates tight junction integrity in human brain endothelial cells
Abstract
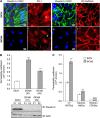
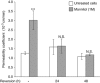
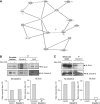
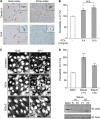
The blood-brain barrier (BBB) selectively controls the exchanges between the blood and the brain: it is formed by tight junctions (TJs) between adjacent microvascular endothelial cells. The transmembrane protein claudin-5 is known as a key TJ protein at the BBB, although, the molecular mechanisms by which it regulates TJ tightness are poorly understood. To identify putative claudin-5 partners that contribute to TJ integrity, claudin-5-enriched membrane microdomains were prepared by cell fractionation, using the human brain endothelial cell line hCMEC/D3 and claudin-5 immunoprecipitates were submitted to tandem mass spectrometry. Because a high concentration of mannitol is known to transiently destabilize TJs, this analysis was performed in basal conditions, after mannitol treatment, and after recovery of TJ integrity. We here demonstrate that the G-protein subunit αi2 (Gαi2) interacts with claudin-5 and that association is correlated with TJ integrity in hCMEC/D3 cells; also, a selective expression of Gαi2 is observed in human brain vasculature in situ. Moreover, small interfering RNA-mediated depletion of Gαi2 or claudin-5 in hCMEC/D3 cells similarly increases their paracellular permeability and delays TJ recovery after mannitol treatment. Altogether, our results identify Gαi2 as a novel claudin-5 partner required for TJ integrity in brain endothelial cells.
Figures
Similar articles
-
A face-to-face comparison of claudin-5 transduced human brain endothelial (hCMEC/D3) cells with porcine brain endothelial cells as blood-brain barrier models for drug transport studies.Fluids Barriers CNS. 2020 Aug 26;17(1):53. doi: 10.1186/s12987-020-00212-5. Fluids Barriers CNS. 2020. PMID: 32843059 Free PMC article.
-
The Wnt/planar cell polarity signaling pathway contributes to the integrity of tight junctions in brain endothelial cells.J Cereb Blood Flow Metab. 2014 Mar;34(3):433-40. doi: 10.1038/jcbfm.2013.213. Epub 2013 Dec 18. J Cereb Blood Flow Metab. 2014. PMID: 24346691 Free PMC article.
-
Reversible opening of the blood-brain barrier by claudin-5-binding variants of Clostridium perfringens enterotoxin's claudin-binding domain.Biomaterials. 2018 Apr;161:129-143. doi: 10.1016/j.biomaterials.2018.01.028. Epub 2018 Feb 2. Biomaterials. 2018. PMID: 29421550
-
Transmembrane proteins of the tight junctions at the blood-brain barrier: structural and functional aspects.Semin Cell Dev Biol. 2015 Feb;38:16-25. doi: 10.1016/j.semcdb.2014.11.004. Epub 2014 Nov 26. Semin Cell Dev Biol. 2015. PMID: 25433243 Review.
-
Holey barrier: claudins and the regulation of brain endothelial permeability.J Cell Biol. 2003 May 12;161(3):459-60. doi: 10.1083/jcb.200304039. J Cell Biol. 2003. PMID: 12743096 Free PMC article. Review.
Cited by
-
iRGD Peptide as a Tumor-Penetrating Enhancer for Tumor-Targeted Drug Delivery.Polymers (Basel). 2020 Aug 24;12(9):1906. doi: 10.3390/polym12091906. Polymers (Basel). 2020. PMID: 32847045 Free PMC article. Review.
-
The long noncoding RNA TUG1 regulates blood-tumor barrier permeability by targeting miR-144.Oncotarget. 2015 Aug 14;6(23):19759-79. doi: 10.18632/oncotarget.4331. Oncotarget. 2015. PMID: 26078353 Free PMC article.
-
Atorvastatin mitigates memory deficits and brain monocyte infiltration in chronic hypercholesterolemia.Aging (Albany NY). 2023 Nov 17;15(23):13669-13679. doi: 10.18632/aging.205217. Epub 2023 Nov 17. Aging (Albany NY). 2023. PMID: 38048213 Free PMC article.
-
DL-3-n-butylphthalide protects the blood-brain barrier against ischemia/hypoxia injury via upregulation of tight junction proteins.Chin Med J (Engl). 2019 Jun 5;132(11):1344-1353. doi: 10.1097/CM9.0000000000000232. Chin Med J (Engl). 2019. PMID: 30939485 Free PMC article.
-
Blood-Brain Barrier Overview: Structural and Functional Correlation.Neural Plast. 2021 Dec 6;2021:6564585. doi: 10.1155/2021/6564585. eCollection 2021. Neural Plast. 2021. PMID: 34912450 Free PMC article. Review.
References
-
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. - PubMed
-
- Adamson P, Wilbourn B, Etienne-Manneville S, Calder V, Beraud E, Milligan G, Couraud PO, Greenwood J. Lymphocyte trafficking through the blood-brain barrier is dependent on endothelial cell heterotrimeric G-protein signaling. FASEB J. 2002;16:1185–1194. - PubMed
-
- Bruckener KE, el Baya A, Galla HJ, Schmidt MA. Permeabilization in a cerebral endothelial barrier model by pertussis toxin involves the PKC effector pathway and is abolished by elevated levels of cAMP. J Cell Sci. 2003;116:1837–1846. - PubMed
-
- Coyne CB, Gambling TM, Boucher RC, Carson JL, Johnson LG. Role of claudin interactions in airway tight junctional permeability. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1166–L1178. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

