Association of influenza virus proteins with membrane rafts
- PMID: 22312341
- PMCID: PMC3265303
- DOI: 10.1155/2011/370606
Association of influenza virus proteins with membrane rafts
Abstract
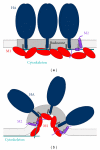
Assembly and budding of influenza virus proceeds in the viral budozone, a domain in the plasma membrane with characteristics of cholesterol/sphingolipid-rich membrane rafts. The viral transmembrane glycoproteins hemagglutinin (HA) and neuraminidase (NA) are intrinsically targeted to these domains, while M2 is seemingly targeted to the edge of the budozone. Virus assembly is orchestrated by the matrix protein M1, binding to all viral components and the membrane. Budding progresses by protein- and lipid-mediated membrane bending and particle scission probably mediated by M2. Here, we summarize the experimental evidence for this model with emphasis on the raft-targeting features of HA, NA, and M2 and review the functional importance of raft domains for viral protein transport, assembly and budding, environmental stability, and membrane fusion.
Figures

Similar articles
-
Lateral Organization of Influenza Virus Proteins in the Budozone Region of the Plasma Membrane.J Virol. 2017 Apr 13;91(9):e02104-16. doi: 10.1128/JVI.02104-16. Print 2017 May 1. J Virol. 2017. PMID: 28202765 Free PMC article.
-
Influenza A virus hemagglutinin and neuraminidase mutually accelerate their apical targeting through clustering of lipid rafts.J Virol. 2014 Sep 1;88(17):10039-55. doi: 10.1128/JVI.00586-14. Epub 2014 Jun 25. J Virol. 2014. PMID: 24965459 Free PMC article.
-
Assembly and budding of influenza virus.Virus Res. 2004 Dec;106(2):147-65. doi: 10.1016/j.virusres.2004.08.012. Virus Res. 2004. PMID: 15567494 Free PMC article. Review.
-
Influenza virus morphogenesis and budding.Virus Res. 2009 Aug;143(2):147-61. doi: 10.1016/j.virusres.2009.05.010. Epub 2009 May 27. Virus Res. 2009. PMID: 19481124 Free PMC article. Review.
-
Acylation and cholesterol binding are not required for targeting of influenza A virus M2 protein to the hemagglutinin-defined budozone.FEBS Lett. 2014 Mar 18;588(6):1031-6. doi: 10.1016/j.febslet.2014.02.014. Epub 2014 Feb 20. FEBS Lett. 2014. PMID: 24561202
Cited by
-
Recombinant H7 hemagglutinin forms subviral particles that protect mice and ferrets from challenge with H7N9 influenza virus.Vaccine. 2015 Sep 11;33(38):4975-82. doi: 10.1016/j.vaccine.2015.07.026. Epub 2015 Jul 21. Vaccine. 2015. PMID: 26207590 Free PMC article.
-
Quantitative analysis of the lipidomes of the influenza virus envelope and MDCK cell apical membrane.J Cell Biol. 2012 Jan 23;196(2):213-21. doi: 10.1083/jcb.201108175. Epub 2012 Jan 16. J Cell Biol. 2012. PMID: 22249292 Free PMC article.
-
Connecting the study of wild influenza with the potential for pandemic disease.Infect Genet Evol. 2013 Jul;17:162-87. doi: 10.1016/j.meegid.2013.02.020. Epub 2013 Mar 26. Infect Genet Evol. 2013. PMID: 23541413 Free PMC article. Review.
-
Influenza A Virus M1 Protein Non-Specifically Deforms Charged Lipid Membranes and Specifically Interacts with the Raft Boundary.Membranes (Basel). 2023 Jan 7;13(1):76. doi: 10.3390/membranes13010076. Membranes (Basel). 2023. PMID: 36676883 Free PMC article.
-
Nothing to sneeze at: a dynamic and integrative computational model of an influenza A virion.Structure. 2015 Mar 3;23(3):584-597. doi: 10.1016/j.str.2014.12.019. Epub 2015 Feb 19. Structure. 2015. PMID: 25703376 Free PMC article.
References
-
- Shishkov AV, Bogacheva EN, Dolgov AA, et al. The in situ structural characterization of the influenza A virus matrix M1 protein within a virion. Protein and Peptide Letters. 2009;16(11):1407–1413. - PubMed
-
- Whittaker GR. Intracellular trafficking of influenza virus: clinical implications for molecular medicine. Expert Reviews in Molecular Medicine. 2001;2001:1–13. - PubMed
-
- Veit M, Schmidt MFG. Palmitoylation of influenza virus proteins. Berliner und Münchener Tierärztliche Wochenschrift. 2006;119(3-4):112–122. - PubMed
-
- Garten W, Klenk HD. Understanding influenza virus pathogenicity. Trends in Microbiology. 1999;7(3):99–100. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

