Regulation of inositol 1,3,4-trisphosphate 5/6-kinase (ITPK1) by reversible lysine acetylation
- PMID: 22308441
- PMCID: PMC3289378
- DOI: 10.1073/pnas.1119740109
Regulation of inositol 1,3,4-trisphosphate 5/6-kinase (ITPK1) by reversible lysine acetylation
Abstract
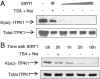
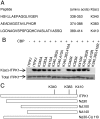
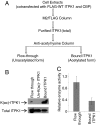
The enzyme inositol 1,3,4-trisphosphate 5/6-kinase (ITPK1) catalyzes the rate-limiting step in the formation of higher phosphorylated forms of inositol in mammalian cells. Because it sits at a key regulatory point in the inositol metabolic pathway, its activity is likely to be regulated. We have previously shown that ITPK1 is phosphorylated, a posttranslational modification used by cells to regulate enzyme activity. We show here that ITPK1 is modified by acetylation of internal lysine residues. The acetylation sites, as determined by mass spectrometry, were found to be lysines 340, 383, and 410, which are all located on the surface of this protein. Overexpression of the acetyltransferases CREB-binding protein or p300 resulted in the acetylation of ITPK1, whereas overexpression of mammalian silent information regulator 2 resulted in the deacetylation of ITPK1. Functionally, ITPK1 acetylation regulates its stability. CREB-binding protein dramatically decreased the half-life of ITPK1. We further found that ITPK1 acetylation down-regulated its enzyme activity. HEK293 cells stably expressing acetylated ITPK1 had reduced levels of the higher phosphorylated forms of inositol, compared with the levels seen in cells expressing unacetylated ITPK1. These results demonstrate that lysine acetylation alters both the stability as well as the activity of ITPK1 in cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

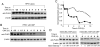
Similar articles
-
Acetylation of sphingosine kinase 1 regulates cell growth and cell-cycle progression.Biochem Biophys Res Commun. 2012 Jan 27;417(4):1242-7. doi: 10.1016/j.bbrc.2011.12.117. Epub 2011 Dec 29. Biochem Biophys Res Commun. 2012. PMID: 22227192
-
Acetylation of WRN protein regulates its stability by inhibiting ubiquitination.PLoS One. 2010 Apr 23;5(4):e10341. doi: 10.1371/journal.pone.0010341. PLoS One. 2010. PMID: 20428248 Free PMC article.
-
Integration of inositol phosphate signaling pathways via human ITPK1.J Biol Chem. 2007 Sep 21;282(38):28117-25. doi: 10.1074/jbc.M703121200. Epub 2007 Jul 6. J Biol Chem. 2007. PMID: 17616525 Free PMC article.
-
The multifaceted role of lysine acetylation in cancer: prognostic biomarker and therapeutic target.Oncotarget. 2016 Aug 23;7(34):55789-55810. doi: 10.18632/oncotarget.10048. Oncotarget. 2016. PMID: 27322556 Free PMC article. Review.
-
Regulation of distinct biological activities of the NF-kappaB transcription factor complex by acetylation.J Mol Med (Berl). 2003 Sep;81(9):549-57. doi: 10.1007/s00109-003-0469-0. Epub 2003 Aug 15. J Mol Med (Berl). 2003. PMID: 12920522 Review.
Cited by
-
Gene editing of three BnITPK genes in tetraploid oilseed rape leads to significant reduction of phytic acid in seeds.Plant Biotechnol J. 2020 Nov;18(11):2241-2250. doi: 10.1111/pbi.13380. Epub 2020 Apr 13. Plant Biotechnol J. 2020. PMID: 32191373 Free PMC article.
-
Activation of PLC by an endogenous cytokine (GBP) in Drosophila S3 cells and its application as a model for studying inositol phosphate signalling through ITPK1.Biochem J. 2012 Dec 1;448(2):273-83. doi: 10.1042/BJ20120730. Biochem J. 2012. PMID: 22928859 Free PMC article.
References
-
- Allfrey VG, Mirsky AE. Structural modifications of histones and their possible role in the regulation of RNA synthesis. Science. 1964;144:559. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

