DC-derived IL-18 drives Treg differentiation, murine Helicobacter pylori-specific immune tolerance, and asthma protection
- PMID: 22307326
- PMCID: PMC3287234
- DOI: 10.1172/JCI61029
DC-derived IL-18 drives Treg differentiation, murine Helicobacter pylori-specific immune tolerance, and asthma protection
Abstract
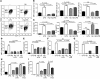
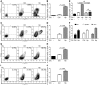
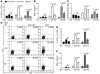
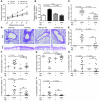
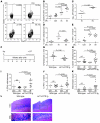
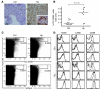
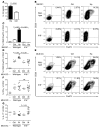
Persistent colonization with the gastric bacterial pathogen Helicobacter pylori causes gastritis and predisposes infected individuals to gastric cancer. Conversely, it is also linked to protection from allergic, chronic inflammatory, and autoimmune diseases. We demonstrate here that H. pylori inhibits LPS-induced maturation of DCs and reprograms DCs toward a tolerance-promoting phenotype. Our results showed that DCs exposed to H. pylori in vitro or in vivo failed to induce T cell effector functions. Instead, they efficiently induced expression of the forkhead transcription factor FoxP3, the master regulator of Tregs, in naive T cells. Depletion of DCs in mice infected with H. pylori during the neonatal period was sufficient to break H. pylori-specific tolerance. DC depletion resulted in improved control of the infection but also aggravated T cell-driven immunopathology. Consistent with the mouse data, DCs infiltrating the gastric mucosa of human H. pylori carriers exhibited a semimature DC-SIGN(+)HLA-DR(hi)CD80(lo)CD86(lo) phenotype. Mechanistically, the tolerogenic activity of H. pylori-experienced DCs was shown to require IL-18 in vitro and in vivo; DC-derived IL-18 acted directly on T cells to drive their conversion to Tregs. CD4(+)CD25(+) Tregs from infected wild-type mice but not Il18(-/-) or Il18r1(-/-) mice prevented airway inflammation and hyperresponsiveness in an experimental model of asthma. Taken together, our results indicate that tolerogenic reprogramming of DCs ensures the persistence of H. pylori and protects against allergic asthma in a process that requires IL-18.
Figures


Comment in
-
Unraveling the mystery of the hygiene hypothesis through Helicobacter pylori infection.J Clin Invest. 2012 Mar;122(3):801-4. doi: 10.1172/JCI61466. Epub 2012 Feb 6. J Clin Invest. 2012. PMID: 22307323 Free PMC article.
Similar articles
-
Helicobacter pylori targets dendritic cells to induce immune tolerance, promote persistence and confer protection against allergic asthma.Gut Microbes. 2012 Nov-Dec;3(6):566-71. doi: 10.4161/gmic.21750. Epub 2012 Aug 16. Gut Microbes. 2012. PMID: 22895083 Free PMC article. Review.
-
Helicobacter pylori immune escape is mediated by dendritic cell-induced Treg skewing and Th17 suppression in mice.Gastroenterology. 2010 Mar;138(3):1046-54. doi: 10.1053/j.gastro.2009.11.043. Epub 2009 Nov 18. Gastroenterology. 2010. PMID: 19931266 Free PMC article.
-
Helicobacter pylori γ-glutamyl transpeptidase and vacuolating cytotoxin promote gastric persistence and immune tolerance.Proc Natl Acad Sci U S A. 2013 Feb 19;110(8):3047-52. doi: 10.1073/pnas.1211248110. Epub 2013 Feb 4. Proc Natl Acad Sci U S A. 2013. PMID: 23382221 Free PMC article.
-
Helicobacter pylori VacA Targets Myeloid Cells in the Gastric Lamina Propria To Promote Peripherally Induced Regulatory T-Cell Differentiation and Persistent Infection.mBio. 2019 Mar 19;10(2):e00261-19. doi: 10.1128/mBio.00261-19. mBio. 2019. PMID: 30890606 Free PMC article.
-
Dendritic cell function in the host response to Helicobacter pylori infection of the gastric mucosa.Pathog Dis. 2013 Feb;67(1):46-53. doi: 10.1111/2049-632X.12014. Epub 2013 Jan 22. Pathog Dis. 2013. PMID: 23620119 Review.
Cited by
-
NOTCH1 is positively correlated with IL17F in Helicobacter pylori infection and a biomarker for mucosal injury.iScience. 2024 Jun 20;27(7):110323. doi: 10.1016/j.isci.2024.110323. eCollection 2024 Jul 19. iScience. 2024. PMID: 39055908 Free PMC article.
-
Complex T cell interactions contribute to Helicobacter pylori gastritis in mice.Infect Immun. 2013 Mar;81(3):740-52. doi: 10.1128/IAI.01269-12. Epub 2012 Dec 21. Infect Immun. 2013. PMID: 23264048 Free PMC article.
-
Immune response modulation in inflammatory bowel diseases by Helicobacter pylori infection.World J Gastroenterol. 2023 Aug 14;29(30):4604-4615. doi: 10.3748/wjg.v29.i30.4604. World J Gastroenterol. 2023. PMID: 37662864 Free PMC article. Review.
-
Decreased risk of celiac disease in patients with Helicobacter pylori colonization.Am J Epidemiol. 2013 Dec 15;178(12):1721-30. doi: 10.1093/aje/kwt234. Epub 2013 Oct 11. Am J Epidemiol. 2013. PMID: 24124196 Free PMC article.
-
Helicobacter hepaticus cholesterol-α-glucosyltransferase is essential for establishing colonization in male A/JCr mice.Helicobacter. 2014 Aug;19(4):280-8. doi: 10.1111/hel.12135. Epub 2014 May 23. Helicobacter. 2014. PMID: 24853076 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

