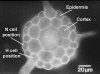
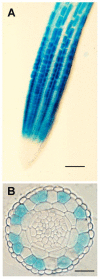
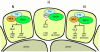
Root hairs
- PMID: 22303213
- PMCID: PMC3243358
- DOI: 10.1199/tab.0060
Root hairs
Figures







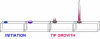

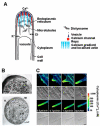

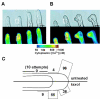

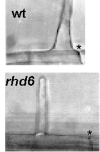



Similar articles
-
The role of root hairs in water uptake: recent advances and future perspectives.J Exp Bot. 2022 Jun 2;73(11):3330-3338. doi: 10.1093/jxb/erac114. J Exp Bot. 2022. PMID: 35323893 Review.
-
The effect of root hairs on root water uptake is determined by root-soil contact and root hair shrinkage.New Phytol. 2023 Dec;240(6):2484-2497. doi: 10.1111/nph.19144. Epub 2023 Jul 31. New Phytol. 2023. PMID: 37525254
-
The root epidermis of Echium plantagineum L.: a novel type of pattern based on the distribution of short and long root hairs.Planta. 2003 Jun;217(2):238-44. doi: 10.1007/s00425-003-0989-4. Epub 2003 Feb 18. Planta. 2003. PMID: 12783331
-
PRX102 Participates in Root Hairs Tip Growth of Rice.Rice (N Y). 2023 Nov 16;16(1):51. doi: 10.1186/s12284-023-00668-7. Rice (N Y). 2023. PMID: 37971600 Free PMC article.
-
Root hairs vs. trichomes: Not everyone is straight!Curr Opin Plant Biol. 2021 Dec;64:102151. doi: 10.1016/j.pbi.2021.102151. Epub 2021 Dec 1. Curr Opin Plant Biol. 2021. PMID: 34864319 Review.
Cited by
-
Bacillus firmus I-1582 promotes plant growth and impairs infection and development of the cyst nematode Heterodera schachtii over two generations.Sci Rep. 2021 Jul 8;11(1):14114. doi: 10.1038/s41598-021-93567-0. Sci Rep. 2021. PMID: 34239009 Free PMC article.
-
Ectopic Expression of JcCPL1, 2, and 4 Affects Epidermal Cell Differentiation, Anthocyanin Biosynthesis and Leaf Senescence in Arabidopsis thaliana.Int J Mol Sci. 2022 Feb 9;23(4):1924. doi: 10.3390/ijms23041924. Int J Mol Sci. 2022. PMID: 35216041 Free PMC article.
-
Optimization of RNA In Situ Hybridization for mRNA Localization Detection in Mature Tissue of Cucumber Seedlings.Plants (Basel). 2020 Oct 29;9(11):1461. doi: 10.3390/plants9111461. Plants (Basel). 2020. PMID: 33138095 Free PMC article.
-
PFT1-controlled ROS balance is critical for multiple stages of root hair development in Arabidopsis.Plant Signal Behav. 2013 May;8(5):e24066. doi: 10.4161/psb.24066. Epub 2013 Mar 1. Plant Signal Behav. 2013. PMID: 23455023 Free PMC article.
-
VPS45 is required for both diffuse and tip growth of Arabidopsis thaliana cells.Front Plant Sci. 2023 Feb 27;14:1120307. doi: 10.3389/fpls.2023.1120307. eCollection 2023. Front Plant Sci. 2023. PMID: 36923123 Free PMC article.
References
-
- Baluska F., Salaj J., Mathur J., Braun M., Jasper F., Samaj J., Chua N. H., Barlow P. W., Volkmann D. Root hair formation: F-actin-dependent tip growth is initiated by local assembly of profilin-supported F-actin meshworks accumulated within expansin-enriched bulges. Dev. Biol. 2000;2271(1):618–632. - PubMed
-
- Bates T. R., Lynch J. P. Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant Cell Environ. 1996;191(1):529–538.
-
- Bates T. R., Lynch J. P. Plant growth and phosphorus accumulation of wild type and two root hair mutants of Arabidopsis thaliana (Brassicaceae). Am. J. Bot. 2000a;871(1):958–963. - PubMed
-
- Bates T. R., Lynch J. P. The efficiency of Arabidopsis thaliana (Brassicaceae) root hairs in phosphorus acquisition. Am. J. Bot. 2000b;871(1):964–970. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
