Noise overexposure alters long-term somatosensory-auditory processing in the dorsal cochlear nucleus--possible basis for tinnitus-related hyperactivity?
- PMID: 22302808
- PMCID: PMC3567464
- DOI: 10.1523/JNEUROSCI.4608-11.2012
Noise overexposure alters long-term somatosensory-auditory processing in the dorsal cochlear nucleus--possible basis for tinnitus-related hyperactivity?
Abstract
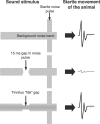
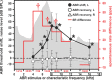
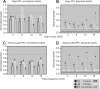
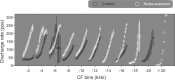
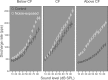
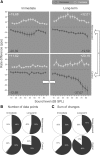
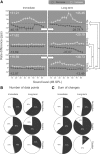
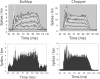
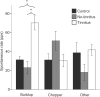
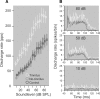
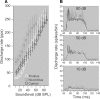
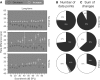
The dorsal cochlear nucleus (DCN) is the first neural site of bimodal auditory-somatosensory integration. Previous studies have shown that stimulation of somatosensory pathways results in immediate suppression or enhancement of subsequent acoustically evoked discharges. In the unimpaired auditory system suppression predominates. However, damage to the auditory input pathway leads to enhancement of excitatory somatosensory inputs to the cochlear nucleus, changing their effects on DCN neurons (Shore et al., 2008; Zeng et al., 2009). Given the well described connection between the somatosensory system and tinnitus in patients we sought to determine whether plastic changes in long-lasting bimodal somatosensory-auditory processing accompany tinnitus. Here we demonstrate for the first time in vivo long-term effects of somatosensory inputs on acoustically evoked discharges of DCN neurons in guinea pigs. The effects of trigeminal nucleus stimulation are compared between normal-hearing animals and animals overexposed with narrow band noise and behaviorally tested for tinnitus. The noise exposure resulted in a temporary threshold shift in auditory brainstem responses but a persistent increase in spontaneous and sound-evoked DCN unit firing rates and increased steepness of rate-level functions. Rate increases were especially prominent in buildup units. The long-term somatosensory enhancement of sound-evoked responses was strengthened while suppressive effects diminished in noise-exposed animals, especially those that developed tinnitus. Damage to the auditory nerve is postulated to trigger compensatory long-term synaptic plasticity of somatosensory inputs that might be an important underlying mechanism for tinnitus generation.
Figures

Similar articles
-
Multi-sensory integration in brainstem and auditory cortex.Brain Res. 2012 Nov 16;1485:95-107. doi: 10.1016/j.brainres.2012.08.037. Epub 2012 Aug 24. Brain Res. 2012. PMID: 22995545 Free PMC article.
-
Noise exposure alters long-term neural firing rates and synchrony in primary auditory and rostral belt cortices following bimodal stimulation.Hear Res. 2017 Dec;356:1-15. doi: 10.1016/j.heares.2017.07.004. Epub 2017 Jul 13. Hear Res. 2017. PMID: 28724501
-
Dorsal cochlear nucleus responses to somatosensory stimulation are enhanced after noise-induced hearing loss.Eur J Neurosci. 2008 Jan;27(1):155-68. doi: 10.1111/j.1460-9568.2007.05983.x. Eur J Neurosci. 2008. PMID: 18184319 Free PMC article.
-
Cross-modal interactions of auditory and somatic inputs in the brainstem and midbrain and their imbalance in tinnitus and deafness.Am J Audiol. 2008 Dec;17(2):S193-209. doi: 10.1044/1059-0889(2008/07-0045). Am J Audiol. 2008. PMID: 19056923 Free PMC article. Review.
-
Tinnitus: Maladaptive auditory-somatosensory plasticity.Hear Res. 2016 Apr;334:20-9. doi: 10.1016/j.heares.2015.06.005. Epub 2015 Jun 12. Hear Res. 2016. PMID: 26074307 Free PMC article. Review.
Cited by
-
Listening to another sense: somatosensory integration in the auditory system.Cell Tissue Res. 2015 Jul;361(1):233-50. doi: 10.1007/s00441-014-2074-7. Epub 2014 Dec 21. Cell Tissue Res. 2015. PMID: 25526698 Free PMC article. Review.
-
Understanding tinnitus: the dorsal cochlear nucleus, organization and plasticity.Brain Res. 2012 Nov 16;1485:40-53. doi: 10.1016/j.brainres.2012.03.044. Epub 2012 Mar 27. Brain Res. 2012. PMID: 22513100 Free PMC article. Review.
-
Cross-Modal Stochastic Resonance as a Universal Principle to Enhance Sensory Processing.Front Neurosci. 2018 Aug 21;12:578. doi: 10.3389/fnins.2018.00578. eCollection 2018. Front Neurosci. 2018. PMID: 30186104 Free PMC article. No abstract available.
-
A Pilot Study of Peripheral Muscle Magnetic Stimulation as Add-on Treatment to Repetitive Transcranial Magnetic Stimulation in Chronic Tinnitus.Front Neurosci. 2018 Feb 20;12:68. doi: 10.3389/fnins.2018.00068. eCollection 2018. Front Neurosci. 2018. PMID: 29515350 Free PMC article.
-
Bimodal stimulus timing-dependent plasticity in primary auditory cortex is altered after noise exposure with and without tinnitus.J Neurophysiol. 2015 Dec;114(6):3064-75. doi: 10.1152/jn.00319.2015. Epub 2015 Aug 19. J Neurophysiol. 2015. PMID: 26289461 Free PMC article.
References
-
- Anari M, Axelsson A, Eliasson A, Magnusson L. Hypersensitivity to sound. Scand Audiol. 1999;28:219–230. - PubMed
-
- Bell CC. Duration of plastic change in a modifiable efference copy. Brain Res. 1986;369:29–36. - PubMed
-
- Bell CC. Memory-based expectations in electrosensory systems. Curr Opin Neurobiol. 2001;11:481–487. - PubMed
-
- Bell CC, Han V, Sawtell NB. Cerebellum-like structures and their implications for cerebellar function. Annu Rev Neurosci. 2008;31:1–24. - PubMed
-
- Bell C, Bodznick D, Montgomery J, Bastian J. The generation and subtraction of sensory expectations within cerebellum-like structures. Brain Behav Evol. 1997;50(Suppl 1):17–31. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

