An endogenous vitamin K-dependent mechanism regulates cell proliferation in the brain subventricular stem cell niche
- PMID: 22290807
- PMCID: PMC3601423
- DOI: 10.1002/stem.1045
An endogenous vitamin K-dependent mechanism regulates cell proliferation in the brain subventricular stem cell niche
Abstract
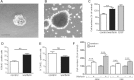
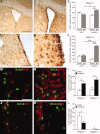
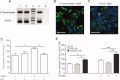
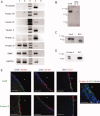
Neural stem cells (NSC) persist in the adult mammalian brain, within the subventricular zone (SVZ). The endogenous mechanisms underpinning SVZ stem and progenitor cell proliferation are not fully elucidated. Vitamin K-dependent proteins (VKDPs) are mainly secreted factors that were initially discovered as major regulators of blood coagulation. Warfarin ((S(-)-3-acetonylbenzyl)-4-hydroxycoumarin)), a widespread anticoagulant, is a vitamin K antagonist that inhibits the production of functional VKDP. We demonstrate that the suppression of functional VKDPs production, in vitro, by exposure of SVZ cell cultures to warfarin or, in vivo, by its intracerebroventricular injection to mice, leads to a substantial increase in SVZ cell proliferation. We identify the anticoagulant factors, protein S and its structural homolog Gas6, as the two only VKDPs produced by SVZ cells and describe the expression and activation pattern of their Tyro3, Axl, and Mer tyrosine kinase receptors. Both in vitro and in vivo loss of function studies consisting in either Gas6 gene invalidation or in endogenous protein S neutralization, provided evidence for an important novel regulatory role of these two VKDPs in the SVZ neurogenic niche. Specifically, we show that while a loss of Gas6 leads to a reduction in the numbers of stem-like cells and in olfactory bulb neurogenesis, endogenous protein S inhibits SVZ cell proliferation. Our study opens up new perspectives for investigating further the role of vitamin K, VKDPs, and anticoagulants in NSC biology in health and disease.
Copyright © 2012 AlphaMed Press.
Figures

Similar articles
-
[Gas-6 and protein S: vitamin K-dependent factors and ligands for the TAM tyrosine kinase receptors family].Med Sci (Paris). 2007 Oct;23(10):826-33. doi: 10.1051/medsci/20072310826. Med Sci (Paris). 2007. PMID: 17937890 Review. French.
-
Evidence for a subventricular zone neural stem cell phagocytic activity stimulated by the vitamin K-dependent factor protein S.Stem Cells. 2015 Feb;33(2):515-25. doi: 10.1002/stem.1862. Stem Cells. 2015. PMID: 25308179
-
Mechanism of inhibitory effect of warfarin on mesangial cell proliferation.J Am Soc Nephrol. 1999 Dec;10(12):2503-9. doi: 10.1681/ASN.V10122503. J Am Soc Nephrol. 1999. PMID: 10589688
-
Gas6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily.FEBS J. 2006 Dec;273(23):5231-44. doi: 10.1111/j.1742-4658.2006.05529.x. Epub 2006 Oct 25. FEBS J. 2006. PMID: 17064312 Review.
-
Vitamin K2 regression aortic calcification induced by warfarin via Gas6/Axl survival pathway in rats.Eur J Pharmacol. 2016 Sep 5;786:10-18. doi: 10.1016/j.ejphar.2016.05.022. Epub 2016 May 19. Eur J Pharmacol. 2016. PMID: 27212383
Cited by
-
GAS6 as a potential target to alleviate neuroinflammation during Japanese encephalitis in mouse models.J Neuroinflammation. 2024 Sep 19;21(1):231. doi: 10.1186/s12974-024-03225-1. J Neuroinflammation. 2024. PMID: 39300526 Free PMC article.
-
Divergent effects of vitamins K1 and K2 on triple negative breast cancer cells.Oncotarget. 2019 Mar 19;10(23):2292-2305. doi: 10.18632/oncotarget.26765. eCollection 2019 Mar 19. Oncotarget. 2019. PMID: 31040920 Free PMC article.
-
Cancer Stem Cells: Emergent Nature of Tumor Emergency.Front Genet. 2018 Nov 16;9:544. doi: 10.3389/fgene.2018.00544. eCollection 2018. Front Genet. 2018. PMID: 30505319 Free PMC article.
-
TAM Receptor Pathways at the Crossroads of Neuroinflammation and Neurodegeneration.Dis Markers. 2019 Sep 15;2019:2387614. doi: 10.1155/2019/2387614. eCollection 2019. Dis Markers. 2019. PMID: 31636733 Free PMC article. Review.
-
Migration/Invasion of Malignant Gliomas and Implications for Therapeutic Treatment.Int J Mol Sci. 2018 Apr 8;19(4):1115. doi: 10.3390/ijms19041115. Int J Mol Sci. 2018. PMID: 29642503 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

