Single vesicle imaging indicates distinct modes of rapid membrane retrieval during nerve growth
- PMID: 22289422
- PMCID: PMC3337222
- DOI: 10.1186/1741-7007-10-4
Single vesicle imaging indicates distinct modes of rapid membrane retrieval during nerve growth
Abstract
Background: During nerve growth, cytoplasmic vesicles add new membrane preferentially to the growth cone located at the distal tip of extending axons. Growth cone membrane is also retrieved locally, and asymmetric retrieval facilitates membrane remodeling during growth cone repulsion by a chemorepellent gradient. Moreover, growth inhibitory factors can stimulate bulk membrane retrieval and induce growth cone collapse. Despite these functional insights, the processes mediating local membrane remodeling during axon extension remain poorly defined.
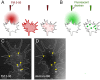
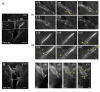
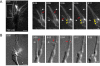
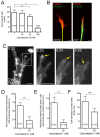
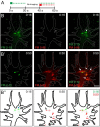
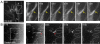
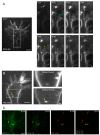
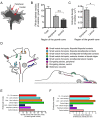
Results: To investigate the spatial and temporal dynamics of membrane retrieval in actively extending growth cones, we have used a transient labeling and optical recording method that can resolve single vesicle events. Live-cell confocal imaging revealed rapid membrane retrieval by distinct endocytic modes based on spatial distribution in Xenopus spinal neuron growth cones. These modes include endocytic "hot-spots" triggered at the base of filopodia, at the lateral margins of lamellipodia, and along dorsal ridges of the growth cone. Additionally, waves of endocytosis were induced when individual filopodia detached from the substrate and fused with the growth cone dorsal surface or with other filopodia. Vesicle formation at sites of membrane remodeling by self-contact required F-actin polymerization. Moreover, bulk membrane retrieval by macroendocytosis correlated positively with the substrate-dependent rate of axon extension and required the function of Rho-family GTPases.
Conclusions: This study provides insight into the dynamic membrane remodeling processes essential for nerve growth by identifying several distinct modes of rapid membrane retrieval in the growth cone during axon extension. We found that endocytic membrane retrieval is intensified at specific subdomains and may drive the dynamic membrane ruffling and re-absorption of filopodia and lamellipodia in actively extending growth cones. The findings offer a platform for determining the molecular mechanisms of distinct endocytic processes that may remodel the surface distribution of receptors, ion channels and other membrane-associated proteins locally to drive growth cone extension and chemotactic guidance.
Figures





Similar articles
-
Bidirectional remodeling of β1-integrin adhesions during chemotropic regulation of nerve growth.BMC Biol. 2011 Nov 30;9:82. doi: 10.1186/1741-7007-9-82. BMC Biol. 2011. PMID: 22126462 Free PMC article.
-
Identification of a developmentally regulated pathway of membrane retrieval in neuronal growth cones.J Cell Sci. 2008 Nov 15;121(Pt 22):3757-69. doi: 10.1242/jcs.033803. Epub 2008 Oct 21. J Cell Sci. 2008. PMID: 18940911 Free PMC article.
-
The Microtubule-Associated Protein Tau Mediates the Organization of Microtubules and Their Dynamic Exploration of Actin-Rich Lamellipodia and Filopodia of Cortical Growth Cones.J Neurosci. 2018 Jan 10;38(2):291-307. doi: 10.1523/JNEUROSCI.2281-17.2017. Epub 2017 Nov 22. J Neurosci. 2018. PMID: 29167405 Free PMC article.
-
Membrane remodeling in clathrin-mediated endocytosis.J Cell Sci. 2018 Sep 3;131(17):jcs216812. doi: 10.1242/jcs.216812. J Cell Sci. 2018. PMID: 30177505 Review.
-
Regulation of growth cone actin filaments by guidance cues.J Neurobiol. 2004 Jan;58(1):92-102. doi: 10.1002/neu.10282. J Neurobiol. 2004. PMID: 14598373 Review.
Cited by
-
Regulation of patterned dynamics of local exocytosis in growth cones by netrin-1.J Neurosci. 2015 Apr 1;35(13):5156-70. doi: 10.1523/JNEUROSCI.0124-14.2015. J Neurosci. 2015. PMID: 25834042 Free PMC article.
-
Red light stimulates vasodilation through extracellular vesicle trafficking.J Photochem Photobiol B. 2021 Jul;220:112212. doi: 10.1016/j.jphotobiol.2021.112212. Epub 2021 May 12. J Photochem Photobiol B. 2021. PMID: 34049180 Free PMC article.
-
Alanine Scanning Mutagenesis of the C-Terminal Cytosolic End of Gpm6a Identifies Key Residues Essential for the Formation of Filopodia.Front Mol Neurosci. 2018 Sep 4;11:314. doi: 10.3389/fnmol.2018.00314. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30233315 Free PMC article.
-
Rab5 and Rab4 regulate axon elongation in the Xenopus visual system.J Neurosci. 2014 Jan 8;34(2):373-91. doi: 10.1523/JNEUROSCI.0876-13.2014. J Neurosci. 2014. PMID: 24403139 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

