Sumatriptan inhibits TRPV1 channels in trigeminal neurons
- PMID: 22289052
- PMCID: PMC3342425
- DOI: 10.1111/j.1526-4610.2011.02053.x
Sumatriptan inhibits TRPV1 channels in trigeminal neurons
Abstract
Objective: To understand a possible role for transient potential receptor vanilloid 1 (TRPV1) ion channels in sumatriptan relief of pain mediated by trigeminal nociceptors.
Background: TRPV1 channels are expressed in small nociceptive sensory neurons. In dorsal root ganglia, TRPV1-containing nociceptors mediate certain types of inflammatory pain. Neurogenic inflammation of cerebral dura and blood vessels in the trigeminal nociceptive system is thought to be important in migraine pain, but the ion channels important in transducing migraine pain are not known. Sumatriptan is an agent effective in treatment of migraine and cluster headache. We hypothesized that sumatriptan might modulate activity of TRPV1 channels found in the trigeminal nociceptive system.
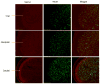
Methods: We used immunohistochemistry to detect the presence of TRPV1 channel protein, whole-cell recording in acutely dissociated trigeminal ganglia (TG) to detect functionality of TRPV1 channels, and whole-cell recording in trigeminal nucleus caudalis (TNC) to detect effects on release of neurotransmitters from trigeminal neurons onto second order sensory neurons. Effects specifically on TG neurons that project to cerebral dura were assessed by labeling dural nociceptors with DiI.
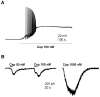
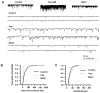
Results: Immunohistochemistry demonstrated that TRPV1 channels are present in cerebral dura, in trigeminal ganglion, and in the TNC. Capsaicin, a TRPV1 agonist, produced depolarization and repetitive action potential firing in current clamp recordings, and large inward currents in voltage clamp recordings from acutely dissociated TG neurons, demonstrating that TRPV1 channels are functional in trigeminal neurons. Capsaicin increased spontaneous excitatory postsynaptic currents in neurons of layer II in TNC slices, showing that these channels have a physiological effect on central synaptic transmission. Sumatriptan (10 µM), a selective antimigraine drug, inhibited TRPV1-mediated inward currents in TG and capsaicin-elicited spontaneous excitatory postsynaptic currents in TNC slices. The same effects of capsaicin and sumatriptan were found in acutely dissociated DiI-labeled TG neurons innervating cerebral dura.
Conclusion: Our results build on previous work indicating that TRPV1 channels in trigeminal nociceptors play a role in craniofacial pain. Our findings that TRPV1 is inhibited by the specific antimigraine drug sumatriptan, and that TRPV1 channels are functional in neurons projecting to cerebral dura suggests a specific role for these channels in migraine or cluster headache.
© 2012 American Headache Society.
Conflict of interest statement
No conflict for any author
Figures



Similar articles
-
Characterization of dural afferent neurons innervating cranial blood vessels within the dura in rats.Brain Res. 2018 Oct 1;1696:91-102. doi: 10.1016/j.brainres.2018.06.007. Epub 2018 Jun 15. Brain Res. 2018. PMID: 29886250
-
Long-Term Depression Induced by Optogenetically Driven Nociceptive Inputs to Trigeminal Nucleus Caudalis or Headache Triggers.J Neurosci. 2018 Aug 22;38(34):7529-7540. doi: 10.1523/JNEUROSCI.3032-17.2018. Epub 2018 Jul 27. J Neurosci. 2018. PMID: 30054391 Free PMC article.
-
Presynaptic inhibition of transient receptor potential vanilloid type 1 (TRPV1) receptors by noradrenaline in nociceptive neurons.J Physiol. 2017 Apr 15;595(8):2639-2660. doi: 10.1113/JP273455. Epub 2017 Feb 22. J Physiol. 2017. PMID: 28094445 Free PMC article.
-
The role of chemosensitive afferent nerves and TRP ion channels in the pathomechanism of headaches.Pflugers Arch. 2012 Sep;464(3):239-48. doi: 10.1007/s00424-012-1142-7. Epub 2012 Aug 9. Pflugers Arch. 2012. PMID: 22875278 Review.
-
Capsaicin, The Vanilloid Receptor TRPV1 Agonist in Neuroprotection: Mechanisms Involved and Significance.Neurochem Res. 2023 Nov;48(11):3296-3315. doi: 10.1007/s11064-023-03983-z. Epub 2023 Jul 26. Neurochem Res. 2023. PMID: 37493882 Free PMC article. Review.
Cited by
-
Genetic and biochemical changes of the serotonergic system in migraine pathobiology.J Headache Pain. 2017 Dec;18(1):20. doi: 10.1186/s10194-016-0711-0. Epub 2017 Feb 13. J Headache Pain. 2017. PMID: 28194570 Free PMC article. Review.
-
No relevant modulation of TRPV1-mediated trigeminal pain by intranasal carbon dioxide in healthy humans.J Headache Pain. 2013 Apr 10;14(1):33. doi: 10.1186/1129-2377-14-33. J Headache Pain. 2013. PMID: 23574808 Free PMC article. Clinical Trial.
-
17β-Estradiol Exacerbated Experimental Occlusal Interference-Induced Chronic Masseter Hyperalgesia by Increasing the Neuronal Excitability and TRPV1 Function of Trigeminal Ganglion in Ovariectomized Rats.Int J Mol Sci. 2021 Jun 28;22(13):6945. doi: 10.3390/ijms22136945. Int J Mol Sci. 2021. PMID: 34203300 Free PMC article.
-
Transient receptor potential channels as targets for phytochemicals.ACS Chem Neurosci. 2014 Nov 19;5(11):1117-30. doi: 10.1021/cn500094a. Epub 2014 Jun 24. ACS Chem Neurosci. 2014. PMID: 24926802 Free PMC article. Review.
-
Inhibitory Gi/O-coupled receptors in somatosensory neurons: Potential therapeutic targets for novel analgesics.Mol Pain. 2018 Jan-Dec;14:1744806918763646. doi: 10.1177/1744806918763646. Mol Pain. 2018. PMID: 29580154 Free PMC article. Review.
References
-
- Montell C, Birnbaumer L, Flockerzi V. The TRP channels, a remarkably functional family. Cell. 2002;108:595–98. - PubMed
-
- Cortright DN, Szallasi A. Biochemical pharmacology of the vanilloid receptor TRPV1. An update. Eur J Biochem. 2004;271:1814–19. - PubMed
-
- Tominaga M, Caterina MJ, Malmberg AB, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–43. - PubMed
-
- Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. - PubMed
-
- Caterina MJ, Leffler A, Malmberg AB, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

