Susceptibility to vaccinia virus infection and spread in mice is determined by age at infection, allergen sensitization and mast cell status
- PMID: 22286752
- PMCID: PMC3291886
- DOI: 10.1159/000330647
Susceptibility to vaccinia virus infection and spread in mice is determined by age at infection, allergen sensitization and mast cell status
Abstract
Background: Patients, especially young children, with atopic dermatitis are at an increased risk of developing eczema vaccinatum, a severe reaction to the smallpox vaccine, either through direct vaccination or indirect contact with a person recently vaccinated.
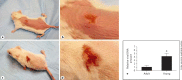
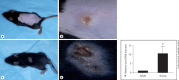
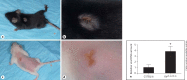
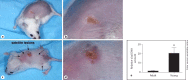
Methods: Using a mouse model of infection, the severity of vaccinia-induced lesions was assessed from their appearance and viral DNA content. The response to vaccinia inoculation was assessed in young and adult mice, allergen-sensitized mice, and in mast cell-deficient mice.
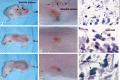
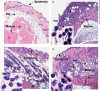
Results: Young age, sensitization to an allergen prior to infection, and a mast cell deficit, accomplished by using mast cell-deficient mice, resulted in more severe viral lesions at the site of inoculation, according to lesion appearance and viral DNA content. All three factors combined demonstrated maximal susceptibility, characterized by the severity of primary lesions and the development of secondary (satellite) lesions, as occurs in eczema vaccinatum in humans. Resistance to the appearance of satellite lesions could be restored by adoptive transfer of bone marrow-derived mast cells from either wild-type or cathelicidin-related antimicrobial peptide-deficient mice. Primary lesions were more severe following the latter transfer, indicating that cathelicidin-related antimicrobial peptide does contribute to the protective activity of mast cells against infection.
Conclusions: The combination of young age, allergen sensitization and a mast cell deficit resulted in the most severe lesions, including satellite lesions. Understanding the factors determining the relative resistance/sensitivity to vaccinia virus will aid in the development of strategies for preventing and treating adverse reactions which can occur after smallpox vaccination.
Copyright © 2012 S. Karger AG, Basel.
Figures
Similar articles
-
Selective killing of vaccinia virus by LL-37: implications for eczema vaccinatum.J Immunol. 2004 Feb 1;172(3):1763-7. doi: 10.4049/jimmunol.172.3.1763. J Immunol. 2004. PMID: 14734759
-
Impaired immune response to vaccinia virus inoculated at the site of cutaneous allergic inflammation.J Allergy Clin Immunol. 2007 Dec;120(6):1382-8. doi: 10.1016/j.jaci.2007.08.004. Epub 2007 Sep 24. J Allergy Clin Immunol. 2007. PMID: 17889291
-
Development of eczema vaccinatum in atopic mouse models and efficacy of MVA vaccination against lethal poxviral infection.PLoS One. 2014 Dec 8;9(12):e114374. doi: 10.1371/journal.pone.0114374. eCollection 2014. PLoS One. 2014. PMID: 25486419 Free PMC article.
-
Adverse events occurring after smallpox vaccination.Semin Pediatr Infect Dis. 2003 Jul;14(3):189-95. doi: 10.1016/s1045-1870(03)00032-3. Semin Pediatr Infect Dis. 2003. PMID: 12913830 Review.
-
Molecular pathogenesis and clinical implications of eczema herpeticum.Expert Rev Mol Med. 2008 Jul 14;10:e21. doi: 10.1017/S1462399408000756. Expert Rev Mol Med. 2008. PMID: 18620613 Review.
Cited by
-
Cationic host defense peptides; novel antimicrobial therapeutics against Category A pathogens and emerging infections.Pathog Glob Health. 2016 Jun-Jul;110(4-5):137-47. doi: 10.1080/20477724.2016.1195036. Epub 2016 Jun 17. Pathog Glob Health. 2016. PMID: 27315342 Free PMC article. Review.
-
Mast Cells Meet Cytomegalovirus: A New Example of Protective Mast Cell Involvement in an Infectious Disease.Cells. 2022 Apr 21;11(9):1402. doi: 10.3390/cells11091402. Cells. 2022. PMID: 35563708 Free PMC article. Review.
-
Age at Vaccination May Influence Response to Sylvatic Plague Vaccine (SPV) in Gunnison's Prairie Dogs (Cynomys gunnisoni).Ecohealth. 2015 Jun;12(2):278-87. doi: 10.1007/s10393-014-1002-3. Epub 2015 Jan 15. Ecohealth. 2015. PMID: 25589000
-
Mast Cell Responses to Viruses and Pathogen Products.Int J Mol Sci. 2019 Aug 30;20(17):4241. doi: 10.3390/ijms20174241. Int J Mol Sci. 2019. PMID: 31480219 Free PMC article. Review.
-
Responses of Mast Cells to Pathogens: Beneficial and Detrimental Roles.Front Immunol. 2021 Jun 15;12:685865. doi: 10.3389/fimmu.2021.685865. eCollection 2021. Front Immunol. 2021. PMID: 34211473 Free PMC article. Review.
References
-
- Esposito JJ, Fenner F. Poxviruses. In: Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 2885–2922.
-
- World Health Organization Declaration of global eradication of smallpox. Wkly Epidemiol Rec. 1980;55:145.
-
- Grabenstein JD, Winkenwerder W. US military smallpox vaccination program experience. JAMA. 2003;289:3278–3282. - PubMed
-
- Rotz LD, Dotson DA, Damon IK, Becher JA. Vaccinia (smallpox) vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2001;50:1–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

