Homeodomain protein otp and activity-dependent splicing modulate neuronal adaptation to stress
- PMID: 22284183
- PMCID: PMC4387198
- DOI: 10.1016/j.neuron.2011.11.019
Homeodomain protein otp and activity-dependent splicing modulate neuronal adaptation to stress
Abstract
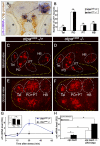
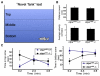
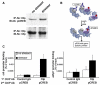
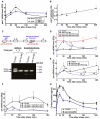
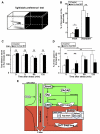
Regulation of corticotropin-releasing hormone (CRH) activity is critical for the animal's adaptation to stressful challenges, and its dysregulation is associated with psychiatric disorders in humans. However, the molecular mechanism underlying this transcriptional response to stress is not well understood. Using various stress paradigms in mouse and zebrafish, we show that the hypothalamic transcription factor Orthopedia modulates the expression of CRH as well as the splicing factor Ataxin 2-Binding Protein-1 (A2BP1/Rbfox-1). We further show that the G protein coupled receptor PAC1, which is a known A2BP1/Rbfox-1 splicing target and an important mediator of CRH activity, is alternatively spliced in response to a stressful challenge. The generation of PAC1-hop messenger RNA isoform by alternative splicing is required for termination of CRH transcription, normal activation of the hypothalamic-pituitary-adrenal axis and adaptive anxiety-like behavior. Our study identifies an evolutionarily conserved biochemical pathway that modulates the neuronal adaptation to stress through transcriptional activation and alternative splicing.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
A vertebrate-conserved cis-regulatory module for targeted expression in the main hypothalamic regulatory region for the stress response.BMC Dev Biol. 2014 Nov 27;14:41. doi: 10.1186/s12861-014-0041-x. BMC Dev Biol. 2014. PMID: 25427861 Free PMC article.
-
Specification of hypothalamic neurons by dual regulation of the homeodomain protein Orthopedia.Development. 2007 Dec;134(24):4417-26. doi: 10.1242/dev.011262. Epub 2007 Nov 14. Development. 2007. PMID: 18003738
-
Homeodomain protein Otp affects developmental neuropeptide switching in oxytocin neurons associated with a long-term effect on social behavior.Elife. 2017 Jan 17;6:e22170. doi: 10.7554/eLife.22170. Elife. 2017. PMID: 28094761 Free PMC article.
-
Stress Adaptation and the Brainstem with Focus on Corticotropin-Releasing Hormone.Int J Mol Sci. 2021 Aug 23;22(16):9090. doi: 10.3390/ijms22169090. Int J Mol Sci. 2021. PMID: 34445795 Free PMC article. Review.
-
Roles for pituitary adenylate cyclase-activating peptide (PACAP) expression and signaling in the bed nucleus of the stria terminalis (BNST) in mediating the behavioral consequences of chronic stress.J Mol Neurosci. 2010 Nov;42(3):327-40. doi: 10.1007/s12031-010-9364-7. Epub 2010 Apr 20. J Mol Neurosci. 2010. PMID: 20405238 Free PMC article. Review.
Cited by
-
Pituitary adenylate cyclase-activating polypeptide receptor activation in the hypothalamus recruits unique signaling pathways involved in energy homeostasis.Am J Physiol Endocrinol Metab. 2022 Mar 1;322(3):E199-E210. doi: 10.1152/ajpendo.00320.2021. Epub 2022 Jan 10. Am J Physiol Endocrinol Metab. 2022. PMID: 35001657 Free PMC article.
-
Toward developmental models of psychiatric disorders in zebrafish.Front Neural Circuits. 2013 Apr 26;7:79. doi: 10.3389/fncir.2013.00079. eCollection 2013. Front Neural Circuits. 2013. PMID: 23637652 Free PMC article. Review.
-
High-resolution tracking of unconfined zebrafish behavior reveals stimulatory and anxiolytic effects of psilocybin.Mol Psychiatry. 2024 Apr;29(4):1046-1062. doi: 10.1038/s41380-023-02391-7. Epub 2024 Jan 17. Mol Psychiatry. 2024. PMID: 38233467 Free PMC article.
-
Probing PAC1 receptor activation across species with an engineered sensor.Elife. 2024 Aug 15;13:RP96496. doi: 10.7554/eLife.96496. Elife. 2024. PMID: 39145773 Free PMC article.
-
Emerging Roles of Alternative Pre-mRNA Splicing Regulation in Neuronal Development and Function.Front Neurosci. 2012 Aug 21;6:122. doi: 10.3389/fnins.2012.00122. eCollection 2012. Front Neurosci. 2012. PMID: 22936897 Free PMC article.
References
-
- Agarwal A, Halvorson LM, Legradi G. Pituitary adenylate cyclase-activating polypeptide (PACAP) mimics neuroendocrine and behavioral manifestations of stress: Evidence for PKA-mediated expression of the corticotropin-releasing hormone (CRH) gene. Brain Res. Mol. Brain Res. 2005;138:45–57. - PMC - PubMed
-
- Aguilera G. Corticotropin releasing hormone, receptor regulation and the stress response. Trends Endocrinol. Metab. 1998;9:329–336. - PubMed
-
- Alsop D, Vijayan MM. Development of the corticosteroid stress axis and receptor expression in zebrafish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R711–R719. - PubMed
-
- Bardet SM, Martinez-de-la-Torre M, Northcutt RG, Rubenstein JL, Puelles L. Conserved pattern of Otp-positive cells in the paraventricular nucleus and other hypothalamic sites of tetrapods. Brain Res. Bull. 2008;75:231–235. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

